Abstract
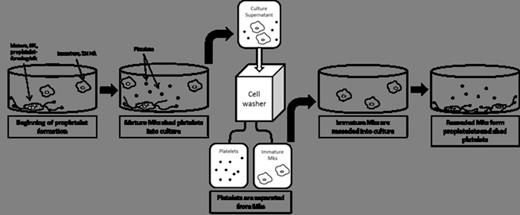
In vitro-derived platelets could supplement volunteer donations for transfusions, while also overcoming problems associated with storage and patient alloimmunization. While platelets generated in vitro from hematopoietic stem and progenitor cells (HSPCs) exhibit similar functional capacity to normal peripheral blood platelets, further advances are needed for this technology to become clinically relevant. One challenge is how to efficiently purify platelets from their precursors, megakaryocytes (Mks), as well as other cell types that arise during heterogeneous differentiation of HSPCs in culture. Recent studies on platelet production from 2D culture have used successive centrifugation to pellet large cells, then platelets, but this multi-step method is subject to error-prone manual manipulation and is unlikely to generate a pure platelet population. As an alternative, we are developing a rapid, one-step approach to separate platelets from larger cells using spinning membrane filtration. Furthermore, because Mk maturation and subsequent platelet release have been shown to occur asynchronously in culture, we have explored how this separation technique could be used to separate platelets and cells at several time points during culture. In this way, one could harvest and prevent pre-activation of platelets generated early in culture, while reseeding immature Mks to generate proplatelets and platelets later in culture.
To first characterize the method, 3 replicative experiments were performed wherein phorbol 12-myristate 13-acetate (PMA)-stimulated (up to 32N) CHRF megakaryoblastic cells were suspended in PAS-V platelet additive solution and mixed with apheresis platelets collected using the Amicus Separator. The mixture of CHRF cells and platelets was processed on a cell washing device consisting of a polycarbonate spinning membrane with 4-µm cylindrical pores. Cells that passed through the membrane (“platelet fraction”) or were retained by the membrane (“cell fraction”) were collected in separate containers. The separation process efficiently recovered platelets in the platelet fraction, while CHRF cells were excluded. Platelets recovered in the platelet fraction showed minimal pre-activation, and CHRF cells recovered in the cell fraction had a similar distribution of viable and apoptotic cells compared to the input population. The ploidy distribution of recovered CHRF cells was also very similar to the input population, suggesting that both smaller and larger cells were eluted from the membrane. Finally, when the recovered CHRF cells were transferred to an adhesive culture surface, they formed proplatelet-like structures similarly to unprocessed cells.
To then apply the method, mobilized peripheral blood CD34+ HSPCs were differentiated to Mks, which formed proplatelets and released platelets in culture. The mixture of in vitro-derived Mks and platelets was then processed on the cell washing device. Similar to the experiments with the CHRF Mk line, primary Mks were excluded from the platelet fraction, and the ploidy distribution of the recovered Mks was similar to the input Mks. Recovered Mks formed fewer proplatelets when reseeded into culture and exhibited increased spreading on the culture surface compared to proplatelet-forming cells observed prior to processing. Since proplatelet formation has been shown to be negatively regulated by Mk contraction caused by engagement of activated integrins, we are exploring whether decreasing the shear rate experienced by Mks during processing may reduce integrin activation and improve proplatelet formation after reseeding. Platelet fraction recovery of CD41+CD42b+ in vitro-derived platelets was lower than that observed with apheresis platelets, and may be attributed to the fact that these larger preplatelets had greater difficulty passing through the 4-µm pores. Recovered in vitro-derived platelets spread in the presence of thrombin similarly to unprocessed in vitro-derived platelets. We are currently exploring how changes in the membrane pore size and shear rate may improve the platelet yield.
| . | . | Pre-Wash . | Post-Wash: Platelet fraction . | Post-Wash: Cell fraction . |
|---|---|---|---|---|
| Platelets | % Recovery | --- | 91 ± 4 | 8 ± 4 |
| % CD62P+, resting | 15 ± 8 | 13 ± 6 | --- | |
| CHRF cells | % Recovery | --- | <0.1 | 74 ± 9 |
| % Viable | 50 ± 3 | --- | 50 ± 7 | |
| % Apoptotic | 29 ± 6 | --- | 26 ± 7 | |
| Mean ploidy | 6.3 ± 0.3 | --- | 6.3 ± 0.6 |
| . | . | Pre-Wash . | Post-Wash: Platelet fraction . | Post-Wash: Cell fraction . |
|---|---|---|---|---|
| Platelets | % Recovery | --- | 91 ± 4 | 8 ± 4 |
| % CD62P+, resting | 15 ± 8 | 13 ± 6 | --- | |
| CHRF cells | % Recovery | --- | <0.1 | 74 ± 9 |
| % Viable | 50 ± 3 | --- | 50 ± 7 | |
| % Apoptotic | 29 ± 6 | --- | 26 ± 7 | |
| Mean ploidy | 6.3 ± 0.3 | --- | 6.3 ± 0.6 |
Table shows average ± SD for 3 experiments with apheresis platelets and CHRF cells.
Radwanski:Fresenius Kabi: Employment. Wegener:Fresenius Kabi: Employment. Min:Fresenius Kabi: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal