Abstract
Thromboembolism is a common complication of nephrotic syndrome (NS). The massive urinary protein loss (proteinuria) of NS results in an acquired, complex hypercoagulopathy. Recent epidemiologic studies have demonstrated that severity of proteinuria in patients with NS is independently predictive for thrombotic risk. Nephrotic-range proteinuria is known to result in acquired deficiencies of antithrombin and free protein S as well as accumulation of procoagulants such as factors V and VIII and von Willebrand factor. However, published cohort studies reveal that the severity of these derangements are quite variable, thus the net effect of proteinuria on thrombotic potential remains unknown. Therefore, we hypothesized that proteinuria severity directly correlates with extent of hypercoagulopathy.
PAN (puromycin aminonucleoside)-induced rat NS is known to induce glomerular injury with peak proteinuria at day ∼9 following intravenous injection. Severity of proteinuria and global hemostasis were compared in five groups of male Wistar rats (body weight ∼150 g) receiving a single i.v. injection of PAN at 0 (saline), 25, 50, 100, or 150 mg/kg (n=4/group). Morning spot urines collected on days 0 (before PAN injection) & 9 were analyzed for urinary [protein]:[creatinine] ratio (uPr:Cr). After urine collection on day 9 the rats were anesthetized with isoflurane and blood was collected from the inferior vena cava into 0.32% NaCitrate/1.45 µM Corn Trypsin Inhibitor [final concentrations]. Rotational thromboelastometry (ROTEM) was performed on whole blood within 20 min of collection, using the INTEM (activated intrinsic pathway) assay with and without urokinase (35 ng). Platelet poor plasma (PPP) was prepared from the remaining blood sample. Thrombin generation assays (Technothrombin TGA) were performed on PPP diluted 1:1 with buffer.
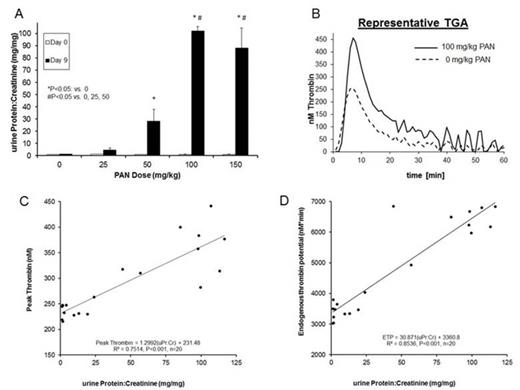
As expected, PAN-treated rats displayed escalating dose-dependent increases in proteinuria at 9 days post-injection (Fig A). The highest dose groups exhibited differential derangements in ROTEM parameters, such that clot formation time (CFT) was decreased and α-angle was increased in rats receiving 100 & 150 mg/kg PAN vs. the sham group (P<0.05; Table). Maximum clot firmness (MCF), amplitude at 10 min (A10) & 20 min (A20), and lysis index at 60 min (LI60) were also significantly higher with the 100 & 150 mg/kg doses, compared to the 0, 25, & 50 groups (P<0.05). Linear regression analysis of uPr:Cr and all measured ROTEM parameters demonstrated that proteinuria was negatively correlated with CFT (R2=0.612, P<0.001), and positively correlated with MCF, A10, A20, & α-angle (respective R2=0.662, R2=0.496, R2=0.536, & R2=0.674; P<0.001 for each parameter). The amount of urokinase-induced fibrinolysis at 60 min was inversely related to proteinuria (LI60; R2=0.674, P<0.001).
TGA parameters also exhibited a dose-dependent effect (Table & Fig B). Peak thrombin and endogenous thrombin potential (ETP) were higher in the 100 & 150 mg/kg dose groups vs. 0, 25 & 50 mg/kg groups (P<0.05). There was a positive correlation between uPr:Cr and parameters of thrombin generation (peak thrombin R2=0.751; ETP R2=0.854; P<0.001; Fig C & D).
These experiments demonstrate that proteinuria severity in the PAN-induced rat NS model is directly proportional to hypercoagulability as assessed by ROTEM and TGA. This suggests that proteinuria may have biologic relevance as an easily measured surrogate marker for hypercoagulopathy severity in NS. Analysis of inducible thrombus formation in PAN-induced rat NS is currently underway to determine the physiologic relevance of these findings.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal