Abstract
The post thrombotic syndrome (PTS) is a chronic complication of deep venous thrombosis (DVT). It is not understood why up to 50% of DVT patients develop PTS despite optimal anticoagulation. In the Bio-SOX Study, we investigated whether markers of inflammation, one of the putative pathophysiologic processes leading to PTS, are of value in predicting the development of PTS in DVT patients.
To assess whether markers of inflammation measured at three time points after DVT diagnosis are predictors of risk of PTS.
The Bio-SOX is a sub-study of the SOX Trial, a multicenter, randomized controlled trial of elastic compression stockings for the prevention of PTS. Eight hundred and three patients with a first, symptomatic, proximal DVT were randomly allocated to receive active or placebo stockings to be worn daily, starting within 2 weeks of DVT diagnosis. During the SOX trial we prospectively collected blood samples from participants at baseline, one and six months. We measured plasma concentrations of C-reactive protein (CRP), interleukin (IL)-6, IL-10 and intercellular adhesion molecule (ICAM)-1 using validated established methods. Differences between median biomarker levels in PTS positive and negative patients (as defined by the Villalta scale applied over time starting at the 6 month visit) were assessed using the Wilcoxon rank-sum test. Multivariable logistic regression models were used to calculate crude and adjusted odds ratios (OR) of PTS by biomarker levels.
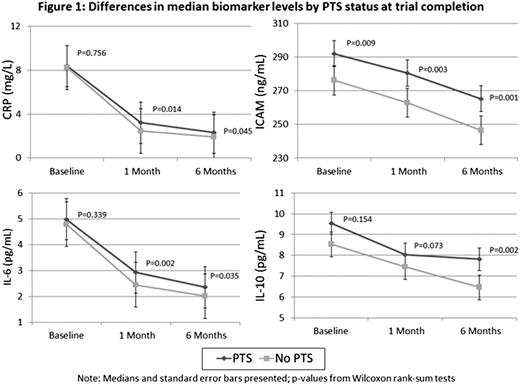
Among study patients, 60.1% were male and mean age was 55.1 years. There were 334 cases of Villalta-defined PTS during follow up, with no significant difference in incidence between the active and placebo stockings groups. Median values of biomarkers between cases and noncases of PTS showed statistically significant differences in CRP and IL-6 at one and six months, ICAM-1 at all time points, and IL-10 at six months post DVT (Figure 1). Table 2 summarizes crude and adjusted ORs for PTS, using the median as a cut-off value. ICAM-1 was associated with PTS at all time points, IL-10 at six months, and CRP at 1 month post DVT, though this association was attenuated after adjustment.
Association between biomarker levels above the median# and PTS.
| . | OR (95% CI) . | ORadjusted (95% CI)* . |
|---|---|---|
| CRP (mg/L) | ||
| Baseline > 8.46 | 1.01 (0.74 – 1.36) | 0.91 (0.66 – 1.25) |
| 1 Month > 2.96 | 1.52 (1.11 – 2.06) | 1.23 (0.88 – 1.72) |
| 6 Months > 2.09 | 1.31 (0.95 – 1.81) | 1.09 (0.76 – 1.55) |
| ICAM-1 (ng/mL) | ||
| Baseline > 285.96 | 1.54 (1.13 – 2.09) | 1.42 (1.03 – 1.97) |
| 1 Month > 275 | 1.63 (1.20 – 2.23) | 1.52 (1.10 – 2.11) |
| 6 Months > 257 | 1.72 (1.24 – 2.37) | 1.60 (1.13 – 2.25) |
| IL-6 (pg/mL) | ||
| Baseline > 4.93 | 1.15 (0.85 – 1.56) | 0.96 (0.69 – 1.34) |
| 1 Month > 2.76 | 1.49 (1.09 – 2.03) | 1.11 (0.79 – 1.57) |
| 6 Months > 2.19 | 1.38 (0.99 – 1.90) | 1.13 (0.79 – 1.63) |
| IL-10 (pg/mL) | ||
| Baseline > 9.18 | 1.26 (0.93 – 1.70) | 1.18 (0.85 – 1.64) |
| 1 Month > 7.78 | 1.30 (0.96 – 1.77) | 1.18 (0.85 – 1.65) |
| 6 Months > 7.09 | 1.78 (1.28 – 2.45) | 1.69 (1.20 – 2.38) |
| . | OR (95% CI) . | ORadjusted (95% CI)* . |
|---|---|---|
| CRP (mg/L) | ||
| Baseline > 8.46 | 1.01 (0.74 – 1.36) | 0.91 (0.66 – 1.25) |
| 1 Month > 2.96 | 1.52 (1.11 – 2.06) | 1.23 (0.88 – 1.72) |
| 6 Months > 2.09 | 1.31 (0.95 – 1.81) | 1.09 (0.76 – 1.55) |
| ICAM-1 (ng/mL) | ||
| Baseline > 285.96 | 1.54 (1.13 – 2.09) | 1.42 (1.03 – 1.97) |
| 1 Month > 275 | 1.63 (1.20 – 2.23) | 1.52 (1.10 – 2.11) |
| 6 Months > 257 | 1.72 (1.24 – 2.37) | 1.60 (1.13 – 2.25) |
| IL-6 (pg/mL) | ||
| Baseline > 4.93 | 1.15 (0.85 – 1.56) | 0.96 (0.69 – 1.34) |
| 1 Month > 2.76 | 1.49 (1.09 – 2.03) | 1.11 (0.79 – 1.57) |
| 6 Months > 2.19 | 1.38 (0.99 – 1.90) | 1.13 (0.79 – 1.63) |
| IL-10 (pg/mL) | ||
| Baseline > 9.18 | 1.26 (0.93 – 1.70) | 1.18 (0.85 – 1.64) |
| 1 Month > 7.78 | 1.30 (0.96 – 1.77) | 1.18 (0.85 – 1.65) |
| 6 Months > 7.09 | 1.78 (1.28 – 2.45) | 1.69 (1.20 – 2.38) |
Median of all patients who had data for that time point
Adjusted for treatment arm (active or placebo stockings), age, sex, body mass index, infectious or inflammatory conditions, congestive heart failure, stroke or myocardial infarction within 1 month prior to DVT, smoking, type of DVT (unprovoked, cancer related, secondary), extent of DVT (popliteal, femoral, common femoral or iliac vein), use of anti-platelet agents, non-steroidal anti-inflammatory drugs, steroids, and statins.
The Bio-SOX Study is the first large scale prospective study we are aware of that evaluated the predictive role of biomarkers measured at baseline and relevant time points after acute DVT in the development of PTS. Findings suggest that inflammation plays an important role in PTS development. Among the studied biomarkers, ICAM-1 appeared to be most strongly associated with PTS development. ICAM-1 is an endothelial- and leukocyte-associated transmembrane protein important in stabilizing cell-cell interactions and facilitating leukocyte endothelial transmigration. Its expression and secretion by activated endothelial cells is induced by thrombin. Future therapeutic trials should be aimed at targeting this pathway.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal