Abstract
The thrombopoietin (TPO) mimetic agents romiplostim and eltrombopag are used to stimulate platelet production and increase platelet counts in chronic immune thrombocytopenia (ITP) in adults. While two large, randomized trials investigating dosing, safety, and efficacy were ongoing in children, but not near completion, we conducted a retrospective IRB-approved analysis of 33 pediatric (≤21 years) chronic ITP patients from two centers treated with TPO agents off-study.
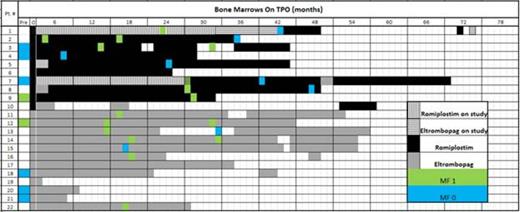
Patients initiated TPO therapy prior to 21 years of age (19 months to 19 years, median 14 years) after an average of 3.6 ITP therapies; 21 patients received romiplostim [11 at Children's Hospital of Orange County (CHOC), 10 at Weill Cornell Medical Center (WCMC)] and 12 eltrombopag [all at WCMC]. Median starting age was 11.5 years for patients on romiplostim and 16.5 years on eltrombopag. Primary response measures were: platelet counts ≥ 50 x 109 per liter or ≥20 x 109 per liter above baseline for 2 consecutive weeks and 50% of platelet counts ≥ 50 x 109 per liter. Duration of treatment and bone marrows with myelofibrosis (MF) consensus grade are shown in Figure 1.
27/33 (82%) patients responded to TPO agents; 18/21 to romiplostim and 9/12 to eltrombopag.
Ten patients responded to ROMIPLOSTIM as a single agent; 8 romiplostim responders received concurrent medications including: mycophenolate mofetil (MMF), azathioprine (AZA), rituximab, and cyclosporine (CSA). One of two splenectomized patients was able to wean off TPO therapy with adequate counts. Two additional patients successfully discontinued TPO therapy and continued on MMF alone. Two patients (1 CSA, 1 AZA) successfully discontinued TPO therapy and then weaned off concurrent therapy.
Eighteen of 21 patients had platelet counts ≥ 50 x 109 per liter or ≥20 x 109 per liter above baseline for 2 consecutive weeks and 18 had 50% of platelet counts ≥ 50 x 109 per liter (table).
In this study, duration of successful romiplostim use outside of randomized clinical trials ranged from 6-44 months (11/18 ongoing). 3 patients did not respond and 2/3 went on to have therapeutic splenectomies. 1 patient with Evans syndrome had a transient 1 year response before relapsing. 5 responders to romiplostim had previously received eltrombopag with lesser or no effect.
| Age of Non – Responders (yrs) . | Plt Ct > 50 x2 weeks . | Plt Ct >20K above baseline . | Plt Ct >50 K for >50% of therapy . | Median Time to Plt >50K (wks) . | Responders w/ Last Ct ≥100K . | |
|---|---|---|---|---|---|---|
| Romiplostim | 4, 5, 8, 9, 17, 17 | 18/21 | 18/21 | 18/21 | 1 | 20/21 |
| Eltrombopag | 3, 10, 15 | 9/12 | 9/12 | 8/12 | 1 | 4/9 |
| Age of Non – Responders (yrs) . | Plt Ct > 50 x2 weeks . | Plt Ct >20K above baseline . | Plt Ct >50 K for >50% of therapy . | Median Time to Plt >50K (wks) . | Responders w/ Last Ct ≥100K . | |
|---|---|---|---|---|---|---|
| Romiplostim | 4, 5, 8, 9, 17, 17 | 18/21 | 18/21 | 18/21 | 1 | 20/21 |
| Eltrombopag | 3, 10, 15 | 9/12 | 9/12 | 8/12 | 1 | 4/9 |
Nine of 12 patients responded to ELTROMBOPAG with platelet counts ≥ 50 x 109 per liter or ≥20 x 109 per liter above baseline for 2 consecutive weeks and 8/12 had 50% of platelet counts ≥ 50 x 109 per liter. One patient had been splenectomized; 7 responded to eltrombopag alone. 2 patients had concurrent therapy with prednisone and IV Anti-D and 1/2 was able to discontinue concurrent prednisone. One patient successfully discontinued eltrombopag with adequate counts for > 1 year. 5 patients attempted unsuccessfully to discontinue therapy. One responder, a previous romiplostim responder, switched to eltrombopag. No other patients were treated with TPO agents prior to starting eltrombopag. Duration of successful eltrombopag use outside of randomized clinical trials ranged from 23-53 months (7/12 are ongoing). Three patients did not respond to eltrombopag; 1/3 was HIV positive. One patient experienced a provoked DVT at site of ankle fracture while using eltrombopag.
No other patients on either therapy experienced serious drug-related adverse events.
Among 24 bone marrows (both agents; 21 reflecting off study use), only MF grades 0 -1 were seen. 10/24 marrows were performed after greater than 2 years of therapy.
Retrospective analysis of off-study use of eltrombopag and romiplostim in children shows that these TPO agents effectively increase platelet counts in more than 3/4 of children with chronic ITP, a result consistent with adult TPO use. Despite small sample size, the long-term duration of successful use (6-53 months) without tachyphylaxis supports efficacy. Re safety, the one DVT appeared to be precipitated by a fracture and there were no MF2/3 bone marrows in the 24 samples. In those patients who do not respond to one TPO agent, it may be beneficial to switch to the other form.
Nugent:Bayer: Honoraria; CSL Behring: Honoraria; Novo Nordisk: Honoraria. Bussel:Shionogi: Membership on an entity’s Board of Directors or advisory committees, Research Funding; Eisai: Membership on an entity’s Board of Directors or advisory committees, Research Funding; Ligand: Membership on an entity’s Board of Directors or advisory committees, Research Funding; Immunomedics: Research Funding; IgG of America: Research Funding; Genzyme: Research Funding; Cangene: Research Funding; GlaxoSmithKline: Equity Ownership, Membership on an entity’s Board of Directors or advisory committees, Research Funding; Amgen: Equity Ownership, Membership on an entity’s Board of Directors or advisory committees, Research Funding; Sysmex: Research Funding; Symphogen: Membership on an entity’s Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal