Abstract
High dose chemotherapy followed by autologous hematopoietic cell transplantation (HCT) is a widely used modality for patients with chemosensitive relapse or with high risk Hodgkin Lymphoma (HL) and non-Hodgkin Lymphoma (NHL). BEAM (carmustine, etoposide, cytarabine, melphalan) is a commonly used preparative regimen; however, for the elderly and for heavily pretreated patients with multiple comorbidities, therapy is hampered by significant regimen-related toxicities. Lower intensity preparative regimen may be less toxic at the potential of reduced efficacy. In this study we compared safety and efficacy profiles between low intensity (loBEAM) and high intensity BEAM (hiBEAM)
We conducted a retrospective sequential-cohort analysis of 143 chemosensitive lymphoma patients treated with BEAM regimen prior to HCT, from January 2005 to June 2013 at two transplant centers in Israel. Fifty-six (39%) patients were treated with hiBEAM (carmustine 300mg/m2, day -6, etoposide 400 mg/m2, days -5 to -2, cytarabine 400 mg/m2, days -5 to -2, and melphalan 140 mg/m2, day -1), and 87 (61%) with loBEAM (any drug dose lower than the above). Diffuse large B cell (DLBCL), mantle-cell (MCL), and T-cell lymphoma (TCL) were defined as aggressive lymphoma. We compared regimen related toxicities, event free and overall survival between the low and high intensity groups. Categorical variables were compared using the chi-square, and the Mann-Whitney non parametric test was used to compare between continuous variables, adjusted to age, when applicable. Overall and progression free survival were estimated using the method of Kaplan and Meier, with cells infusion day serving as day 0.
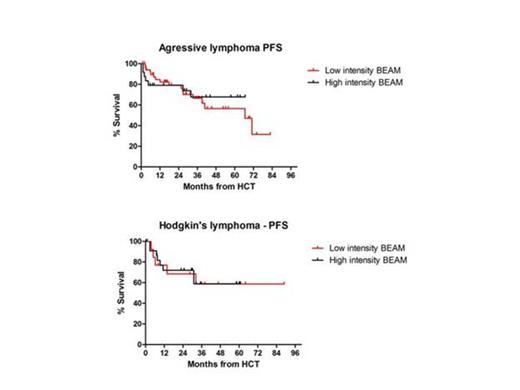
Median age of patients was 36 (range, 19-58) years for hiBEAM cohort, and 58 (range, 18-78) years for the loBEAM group (p<0.001). Median follow up for all cohort was 39 (range, 2-93) months. Forty-eight patients had HL (34%), 39 (27%) had DLBCL, 24 (17%) had TCL, 19 (13%) had MCL, and 13 (9%) patients had low grade lymphoma. Disease status prior to HCT was similar between the two groups with 52 (36%) patients in first complete remission (CR1), and 90 (63%) patients in CR2/CR3, or in partial remission (p=0.37),. Among the patients given loBEAM the mean cumulative dose was 71% (SD ±11%). Median time to neutrophil engraftment was statistically significant shorter in patients given loBEAM compared those given hiBEAM (9 vs. 10 days, p<0.001). There were significantly lower incidences of grade 3-4 mucositis and elevated liver enzymes at the loBEAM group compared to the hiBEAM group (49% vs. 66%, p=0.05 and 9% vs. 27%, p=0.009, respectively). There were no differences between the two groups in the incidences of grade 3-4 renal dysfunction and non hematologic toxicities and no difference in the incidences of microbiology documented infections. Progression free survival (PFS) at 3 years were comparable between hiBEAM and loBEAM, 75% and 70% respectively, p=0.27. Subgroup analyses for patients with HL and aggressive lymphoma showed similar for HL patients, 3-years PFS were 59% for both hiBEAM and loBEAM (p=0.93). For the aggressive lymphoma group, 3-years PFS were 67% vs. 66%, p=0.73, respectively (Figure). Non relapse mortality at day 100 post transplantation was similar in both groups (3.6% vs. 3.4%, p=1.0, respectively). Three-year overall survivals were similar for hiBEAM and loBEAM in patients with HL (73% and 92%, respectively, p=0.28) and in patients with aggressive lymphoma (78% and 68%, respectively, p=0.38).
Dose reduced BEAM conditioning prior to HCT for patients with chemosensitive lymphoma has lower toxicity compared to high dose BEAM, while maintaining similar efficacy. This enables utilizing HCT for both elderly and patients with significant comorbidities without diminish HCT benefit. Positive outcomes of such studies could pave way for better treatment strategies for these patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal