Abstract
The order of alternative donor selection for hematopoietic stem cell transplantation (HSCT) for patients with hematologic malignancies has not been addressed. We performed the first prospective trial to compare the effect of HSCT from matched sibling donors (MSDs), unrelated donors (URDs) and haploidentical- related donors (HRDs) in a contemporary protocol.
From 2008 to 2012, 234 patients with hematologic malignancies were enrolled. The treatment schedule was as follows: if a fully MSD was available, patients were assigned treatment with MSD-HSCT. If an MSD was unavailable, a suitably matched URD was used as the alternative, where a suitable match involved matching more than 8 of 10 HLA-A, -B, -C, -DRB1and DQ allele loci ( ¡Ý 8/10) and at least 5 of 6 matching HLA-A, -B, and -DRB1 antigen loci. If only URDs with > 2 mismatching allele loci were available, patients were allowed treatment with HRD-HSCT.
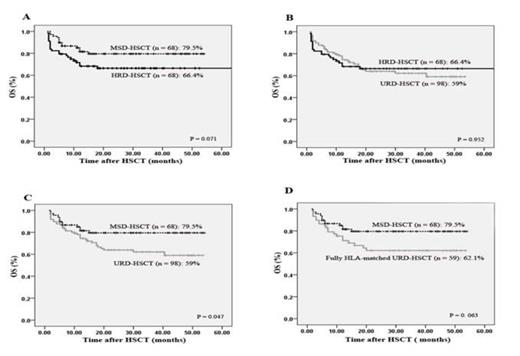
(1) Sixty-eight patients underwent MSD-HSCT, 98 patients underwent URD-HSCT, and 68 patients underwent HRD-HSCT (Table 1). (2) Grades II¨CIV and severe aGVHD were all significantly more frequent in patients undergoing HRD-HSCT compared with those undergoing MSD-HSCT (II¨CIV: 42.6% vs 19.1%, P = 0.0015; severe aGVHD: 17.65% vs 5.88%, P = 0.03). However, the incidences of II¨CIV and severe aGVHD were comparable in patients receiving transplants from HRDs to those from URDs (II¨CIV: 42.6% vs 40.8%, P = 0.89; severe aGVHD: 17.65% vs 13.27%, P = 0.48). The incidence of cGVHD was not significantly affected by donor types. (3) The 4-year incidence of relapse was not significantly affected by donor types according to all patients (24.2% in the MSD cohort, 22.8% in the URD cohort, 11.9% in the HRD cohort, P > 0.05). However, after controlling for high-risk patients, a superior graft-versus-leukemia (GVL) effect was observed in patients undergoing HRD-HSCT compared to MSD-HSCT or URD-HSCT. In high-risk patients receiving MSD, 36.8% experienced relapse, as did 33.6% in the URD cohort, but the incidence decreased to11.1% in the HRD cohort (MSD vs HRD, P = 0.015; URD vs HRD, P = 0 .028). (4) HRD-HSCT yielded comparable rates of 4-year overall survival (OS) and disease-free survival (DFS) to MSD-HSCT ( OS: 66.4% vs 79.5%, P = 0.071; DFS: 66.4% vs 78.2 %, P = 0.109) or URD-HSCT (OS: 66.4% vs 59%, P = 0.952; DFS: 66.4% vs 58.1%, P = 0.864) (Figure 1).
Patient-, disease-, and transplantation-related characteristics
| Characteristics | MSD HSCT ( n=68 ) | URD HSCT ( n=98 ) | HRD HSCT ( n=68 ) | P value | ||
| MSD vs URD | MSD vs HRD | URD vs HRD | ||||
| Age (median, range), years | 32.5 (16-56) | 26 (10-50) | 25 (9-55) | < 0.001 | < 0.001 | NS |
| Underlying disease, n (%) | NS | NS | NS | |||
| AML | 29 (42.6) | 38 (38.8) | 20 (29.4) | |||
| ALL | 24 (35.3) | 44 (44.9) | 31(45.6) | |||
| MPAL | 0 | 2 (2) | 2 (2.9) | |||
| CML | 5 (7.4) | 2 (2) | 5 (7.4) | |||
| MDS | 6 (8.8) | 8 (8.2) | 7 (10.3) | |||
| NHL | 4 (5.9) | 4 (4.1) | 3 (4.4) | |||
| Donor-patient gender, n(%) | 0.002 | NS | 0.001 | |||
| Male-male | 15 (22.1) | 47 (48) | 23 (33.8) | < 0.001 | NS | 0.005 |
| Male-female | 14 (20.6) | 26 (26.5) | 13 (19.1) | |||
| Female-male | 21 (30.9) | 11 (11.2) | 23 (33.8) | |||
| Female-female | 18 (26.5) | 14 (14.3) | 9 (13.2) | |||
| Donor-patient blood group, n(%) | < 0.001 | NS | 0.002 | |||
| Identical | 45 (66.2) | 36 (36.7) | 42 (61.8) | |||
| Incompatibility | 23 (33.8) | 62 (63.3) | 26 (38.2) | |||
| Risk classification, n (%) | NS | NS | NS | |||
| Low risk | 1 (1.5) | 2 (2) | 0 (0) | |||
| Intermediate/Standard | 29 (42.6) | 38(38.8) | 20 (29.4) | |||
| High risk | 38 (55.9) | 58(59.2) | 48 (70.6) | |||
| Time from diagnosis to HSCT, mo | NS | NS | NS | |||
| Median (range) | 7.5 (3-126) | 9 (3-108) | 8.5 (3-144) | |||
| Disease status at HSCT, n (%) | NS | NS | NS | |||
| CR1 | 54 (79.4) | 74 (75.5) | 47 (69.1) | |||
| CR2 | 8 (11.8) | 13 (13.3) | 15 (22.1) | |||
| ¡Ý CR3 | 1 (1.5) | 4 (4.1) | 0 (0) | |||
| Advanced stage | 5(7.4) | 7(7.1) | 6 (8.8) | |||
| Median MNCs, ¡Á108/kg (range) | 9.96 (0.84-18.66) | 9.76 (2.37-27.25) | 10.53 (2.86-16.12) | NS | NS | NS |
| Median CD34+ count, ¡Á106/kg (range) | 3.68 (0.715-14.1) | 5 (0.86-29.64) | 3.05 (0.8-14.13) | 0.003 | NS | < 0.001 |
| Median follow-up for patients, mo (range) | 27 (5-54) | 31 (5-53.5) | 27 (5.5-52.5) | NS | NS | NS |
| Characteristics | MSD HSCT ( n=68 ) | URD HSCT ( n=98 ) | HRD HSCT ( n=68 ) | P value | ||
| MSD vs URD | MSD vs HRD | URD vs HRD | ||||
| Age (median, range), years | 32.5 (16-56) | 26 (10-50) | 25 (9-55) | < 0.001 | < 0.001 | NS |
| Underlying disease, n (%) | NS | NS | NS | |||
| AML | 29 (42.6) | 38 (38.8) | 20 (29.4) | |||
| ALL | 24 (35.3) | 44 (44.9) | 31(45.6) | |||
| MPAL | 0 | 2 (2) | 2 (2.9) | |||
| CML | 5 (7.4) | 2 (2) | 5 (7.4) | |||
| MDS | 6 (8.8) | 8 (8.2) | 7 (10.3) | |||
| NHL | 4 (5.9) | 4 (4.1) | 3 (4.4) | |||
| Donor-patient gender, n(%) | 0.002 | NS | 0.001 | |||
| Male-male | 15 (22.1) | 47 (48) | 23 (33.8) | < 0.001 | NS | 0.005 |
| Male-female | 14 (20.6) | 26 (26.5) | 13 (19.1) | |||
| Female-male | 21 (30.9) | 11 (11.2) | 23 (33.8) | |||
| Female-female | 18 (26.5) | 14 (14.3) | 9 (13.2) | |||
| Donor-patient blood group, n(%) | < 0.001 | NS | 0.002 | |||
| Identical | 45 (66.2) | 36 (36.7) | 42 (61.8) | |||
| Incompatibility | 23 (33.8) | 62 (63.3) | 26 (38.2) | |||
| Risk classification, n (%) | NS | NS | NS | |||
| Low risk | 1 (1.5) | 2 (2) | 0 (0) | |||
| Intermediate/Standard | 29 (42.6) | 38(38.8) | 20 (29.4) | |||
| High risk | 38 (55.9) | 58(59.2) | 48 (70.6) | |||
| Time from diagnosis to HSCT, mo | NS | NS | NS | |||
| Median (range) | 7.5 (3-126) | 9 (3-108) | 8.5 (3-144) | |||
| Disease status at HSCT, n (%) | NS | NS | NS | |||
| CR1 | 54 (79.4) | 74 (75.5) | 47 (69.1) | |||
| CR2 | 8 (11.8) | 13 (13.3) | 15 (22.1) | |||
| ¡Ý CR3 | 1 (1.5) | 4 (4.1) | 0 (0) | |||
| Advanced stage | 5(7.4) | 7(7.1) | 6 (8.8) | |||
| Median MNCs, ¡Á108/kg (range) | 9.96 (0.84-18.66) | 9.76 (2.37-27.25) | 10.53 (2.86-16.12) | NS | NS | NS |
| Median CD34+ count, ¡Á106/kg (range) | 3.68 (0.715-14.1) | 5 (0.86-29.64) | 3.05 (0.8-14.13) | 0.003 | NS | < 0.001 |
| Median follow-up for patients, mo (range) | 27 (5-54) | 31 (5-53.5) | 27 (5.5-52.5) | NS | NS | NS |
OS at 4 years after transplantation stratified according to the donor types.
Our data provide convincing clinical evidence to support the use of HRDs, as well as URDs, can be selected as first-line alternative donors, especially for high-risk patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal