Abstract
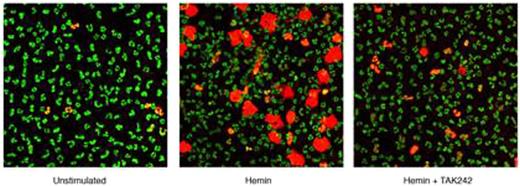
PMN and eosinophils (EO) activated by physiologic agonist such as LPS, C5a, GM-CSF and IL-5 form extracellular traps (ET) comprised of extracellular DNA, histones, and active secondary granule constituents that retain microbicidal capacity and thus contribute to host defense but also participate in the pathogenesis of sepsis, microangiopathy, vasculitis, asthma and thrombosis. Previous studies have implicated superoxide, H2O2 and myeloperoxidase (MPO) in PMN ET formation, which reflects a novel death pathway dubbed “ETosis.” Thiocyanate (SCN-) is the principle physiologic substrate for eosinophil peroxidase (EPO) and a major substrate for MPO. Because previous studies of ET were all performed in the absence of SCN- and HOSCN, the product of SCN- peroxidation, is a weak, sulfhydryl-specific oxidant markedly less toxic than HOCl or HOBr, we hypothesized SCN- blocks PMN and EO ET formation. We also hypothesized that heme, elevated levels of which occur in intravascular hemolytic states such as sickle cell disease, induces PMN ETosis because both LPS and heme signal by engaging TLR4. We assessed ET formation by human PMN stimulated with either GM-CSF + C5a or 10 µM heme in RPMI one hour using glass slide-attached leukocytes with both cell impermeant (Sytox Orange) and permeant (Syto13) DNA stains and IF localization of secondary granule proteins using confocal microscopy. ET formation in response to to both stimuli was 5-20% of PMN in stimulated cells (vs. 0% in control). The NADPH oxidase inhibitor DPI, MPO inhibitor 4-ABAH and 1 mM SCN- all diminished ET formation by >80% in response to both stimuli. Heme-dependent ET formation was inhibited 80% by the TLR4 antagonist TAK242 (see figure below in which intracellular DNA stains green and extracellular and membrane-compromised PMN DNA stains orange). PMA-induced PMN ET formation requires singlet O2 resulting from the secondary reaction of HOCl with excess H2O2. In PMN stimulated with GM-CSF + C5a, the singlet O2 scavenger edaravone (10 µM) inhibited ETs by 80%. ETs also formed in 4-8% of EOs stimulated by IL-5 + C5a, vs. 0% in control EO and 22.5% in unstimulated EO supplemented with the alternative EPO substrate 1 mM Br-. The EPO inhibitor resorcinol decreased by >90% EO ET formation stimulated with by IL-5 + C5a or by adding Br-, as did addition of SCN- or edaravone. These studies show that both PMN and EO ETosis depend upon MPO- and EPO- generated oxidants, respectively, and the presence of an alternative physiologic substrate, SCN-, markedly antagonizes ETosis. We propose that SCN- inhibits ET formation by generating HOSCN that reacts with H2O2 to yield cyanate, not singlet O2. In addition, we also demonstrate that heme is a potent inducer of PMN ETosis through a mechanism dependent upon TLR4, NADPH oxidase and MPO, raising the possibility that this pathway may directly promote the inflammation and thrombosis that contribute of the pathogenesis of sickle cell disease. Based on these findings, we speculate that dietary augmentation of SCN- to normal or supranormal levels might have clinically beneficial therapeutic effects in a variety of inflammatory states, including sickle cell disease.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal