Abstract
Bortezomib has been shown to produce an anabolic bone effect (increase bone ALP and osteocalcin) in relapsed/refractory patients. This study examined the bone anabolic effect in patients with smoldering multiple myeloma (SMM) who, with a historical median age of 67 years, have frequent evidence of osteopenia not associated with lytic bone disease. SMM is usually followed expectantly without therapy. The overall risk of progression to active MM has been estimated up to 20% in the first year from diagnosis (Kyle et al, 2007). The primary aim of this trial is to determine the effect of a course of low-dose Bortezomib on bone remodeling and on disease progression.
The dose of bortezomib used in this trial of 0.7mg/m² is the lowest dose which has shown efficacy in the 3 largest monotherapy trials with bortezomib. Patients enrolled in this study had serum M protein ≥ 3 g/dL and/or bone marrow plasma cells ≥ 10% with absence of anemia, renal failure, hypercalcemia, and lytic bone lesions. Patients received 9 cycles of bortezomib given on days 1, 8, 15, 22 every 42 days. No bisphosphonates were allowed during the trial, Vitamin D supplements were allowed.
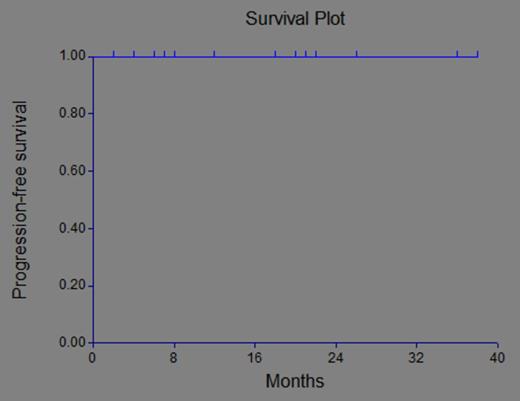
Seventeen patients (9 males) with a median age of 61 years were enrolled in the study. Fourteen had IgG paraprotein and 3 had an IgA. Two participants did not complete the treatment; the first because of development of a skin rash and the second for personal reasons. A total of 11 patients completed the protocol. Four are still receiving treatment. At the time of this analysis with a median follow-up of 20 months, all patients are alive and none has progressed (Figure 1). The mean M component at baseline, end-of-study, and at most recent follow-up are shown in Figure 2. The treatment was well tolerated by the patients. One patient developed reversible grade 3 neuropathy and a drug related rash and only 3 grade 3 hematological adverse events were recorded (15%). Bone densities by DEXA scan were obtained at baseline, end of study, and yearly thereafter. Out of 17 patients, 13 had bone density T-scores before and after treatment. Six patients (46%) showed an improvement in hip T-score (mean T-score improvement 0.41, range 0.1-1.35). In the overall group, mean T scores improved by 38% (range, 3-200%). T score in lumbar spine improved in 3 patients (23%), who had a mean T-score improvement of 0.2 (range, 0.05-0.43).
The use of low dose Velcade in smoldering myeloma patients was well tolerated and produces significant increases in bone density in 46% of participants.
Zangari: Millenium Pharm.: Research Funding. Off Label Use: Use of Bortezomib in smoldering myeloma. Salama:Eli Lilly and Co: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal