Abstract
Despite of recent advances in therapeutic strategy for multiple myeloma (MM), MM still remains incurable and novel therapeutic approach is urgently needed. We have previously demonstrated that a natural small molecule, shikonin (SHK), induced both apoptosis and necroptosis (programmed necrosis) in MM cells. In this study, we attempted to elucidate biological mechanisms of SHK in inducing apoptosis and necroptosis.
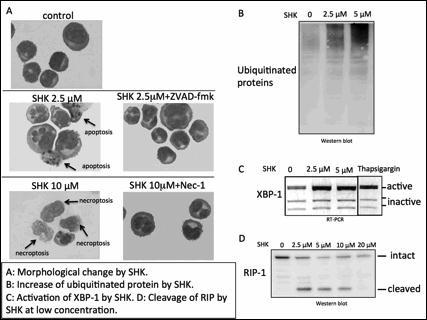
Six MM cell lines, KMS-12-PE, RPMI 8226, U266, KMM1, KMS-11 and a bortezomib-resistant MM cell line, KMS-11/BTZ (obtained from Kyowa Hakko Kirin Co. Ltd.), were utilized. Inhibitors of pan-caspase and necroptosis, ZVAD-fmk and Nec-1 (necrostatin-1), were employed to distinguish apoptosis and necroptosis, respectively. Cell death was analyzed using the trypan blue dye exclusion method (WST-8 assay) and flow cytometry analysis using AnnexinV/PI staining. Morphological examinations of cells were performed with May Giemza staining. Caspases, RIP1, ubiquitinated proteins, and heat shock proteins were analyzed with western blot. Knockdown of RIP1, an essential molecule for necroptosis, was performed using siRNA. ER stress was assessed by detecting activated XBP-1, which was analyzed by digestion of PCR products with ApaLI. Because the ApaLI site in XBP-1 mRNA is spliced out upon activation, the activated XBP-1 shows one large band after ApaLI digestion, while inactivated XBP-1 shows two ApaI-digested bands. Thapsigargin, sarcoplasmic/endoplasmic reticulum Ca2+ ATPase (SERCA) inhibitor, was used as an ER stress inducer.
By screening natural compounds libraries (provided by Institute of Natural Medicine, Toyama University, Japan), we found that SHK, a natural compound derived from the root of Lithospermum erythrorhizon, induced cell death in MM cells. Apoptosis was induced at a relatively low concentration (2.5∼5 µM) and was inhibited by a caspase-inhibitor, while necroptosis was promptly induced at higher concentrations (10∼ 20 µM) within 5 hours and was completely inhibited by Nec-1. Morphological analysis showed that SHK at low concentrations induced typical apoptotic changes, such as fragmented nucleus, while SHK at higher concentrations induced necrotic morphology, such as translucent cytoplasm and swelling of cell membranes. By contrast, SHK did not induce apoptosis or necrptosis in peripheral blood mononuclear cells from healthy donors at low concentrations. SHK activated caspase-8 and -3 at low concentrations but did not at higher concentrations. RIP1, an essential molecule for necroptosis, was cleaved after treatment with SHK at low concentrations, which leads to the inhibition of necroptosis, while it was not cleaved and remained active at higher concentrations, suggesting that SHK dynamically regulates the cleavage of RIP-1.
At low concentrations, shikonin induced an accumulation of ubiquitinated proteins and activated XBP-1, suggesting SHK may have a property of proteasome inhibitor eventually inducing endoplasmic reticulum stress. Finally, SHK at low concentration killed bortezomib resistant cells with lower IC50 comparing to that of the parental cells (0.91 vs 1.56 µM, respectively).
We here report, for the first time, that SHK induces apoptosis and necroptosis in MM cells at low and high concentrations, respectively, by regulating proteasome function and RIP-1 cleavage. Given the fact that SHK efficiently induces cell death in bortezomib-resistant cell line, SHK may act as a novel proteasome inhibitor for bortezomib-resistant myeloma cells. Moreover, SHK at higher concentrations, which induces nectoptosis, should be an attractive future therapeutic option potentially to eradicate MM cells.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal