DLBCL patients (pts) with an intermediate/high to high risk international prognostic index (IPI) are at an increased risk of disease relapse rate in the first year after completion of standard therapy with R-CHOP. Lenalidomide (len), an immunomodulatory drug, has activity in relapsed diffuse large B cell Lymphoma. Len enhances the natural-killer cell mediated antibody-dependant cellular cytotoxicity of rituximab in lymphoma cell lines and inhibits angiogenesis as well as alters cytokine production. Lenalidomide received FDA approval based on its clinical activity in relapsed mantle cell lymphoma.
Intermediate-high/high risk IPI pts with DLBCL were randomized to len (arm A) alone or len and rituximab (arm B). The primary endpoint of the study was to assess the one year disease-free survival. We expected that a 25% difference of relapse will have clinical significance when compared with current standard therapy. Pts in arm A received len at a dose of 25 mg daily for 21 days of 28 days. Patients on arm B received len at a dose of 20mg daily for 21 days of 28 days along with rituximab on day 8 of even cycles. Treatment on both arms was continued for one year. Treatment was discontinued for disease progression. Len dose adjustments are incorporated in the protocol.
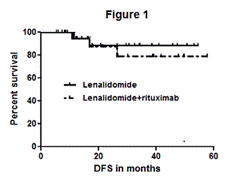
Forty three pts, 21 arm A/ 22 arm B, 21 female/22 male, with a median age of 59 yrs were enrolled. The median IPI was 4 for pts over the age of 60 and the median aa-IPI was 3. Three pts received XRT to areas of bulky disease. At a median follow up of 27 months, the 2 yr DFS and OS was 88%. For patients in arm A and arm B the 2 yr DFS was 90% vs. 86% (figure 1) and the 2 yr OS was 95.2% vs. 81% (figure 2), respectively (P=NS). Two pts discontinued treatment due to adverse events. Grade 3-4 toxicities include neutropenia (28%), fatigue (16%), diarrhea (6%), DVT (3%), rash (3%), febrile neutropenia (3%). Related grade 1-2 toxicities include hypothyroidism (15%) and rash (54%).
Len as maintenance therapy demonstrates clinical activity following standard chemotherapy and improves DFS and OS in DLBCL patients with high risk prognostic features as compared with historical controls. This trial is registered with NCI- #NCT00765245
Reddy:Celgene: Research Funding. Off Label Use: lenalidomide in diffuse large B cell lymphoma. Park:TEVA: Research Funding; Seattle Genetics, Inc.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal