Abstract
Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) has an indolent clinical course, with overall patient survival at 5 years after diagnosis of more than 85%. Although the cause of death in a significant proportion of MALT lymphoma patients is not the lymphoma itself, some patients experience early relapse or refractory disease. However, the risk factors capable of predicting aggressive disease in MALT lymphoma have not been clearly defined.
This is a retrospective pooled analysis of pathologically confirmed extranodal MALT lymphoma patients treated at our institute. Patients with nodal or splenic marginal zone lymphoma were excluded. The primary endpoint was progression-free survival (PFS), which was assessed using the Kaplan-Meier method. The log-rank test and multivariate Cox regression analysis were used to assess the prognostic value of each clinical variable.
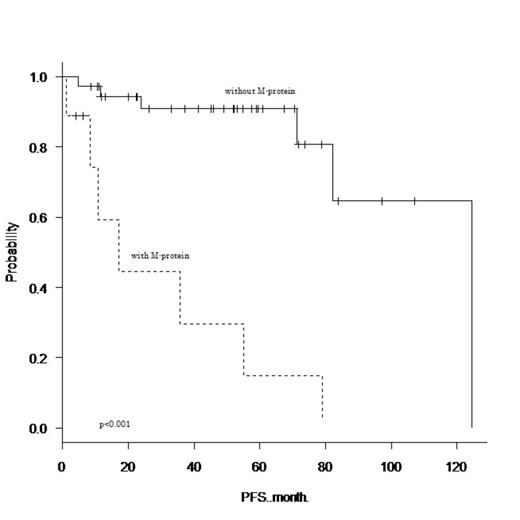
From January 2000 to June 2013, 52 extranodal MALT lymphoma patients (26 females and 26 males) were referred to our institution. The median age of the patients was 68 years (range, 43–89 years). Thirty-three (63%) patients had limited stage disease (stage I/II) and 48 (92%) patients had an ECOG performance status of less than 2. The cumulative numbers of patients who were diagnosed with orbit, thyroid, salivary gland, stomach, lung, and intestine disease were 7 (13%), 5 (10%), 9 (17%), 16 (31%), 16 (31%), and 4 (8%), respectively. Fifteen (29%) patients had a prior history of autoimmune disease, and a serum electrophoresis study detected M-protein in 9 (17%) patients (5 patients had IgG, 3 patients had IgM, and 1 patient had IgA). There was no significant correlation between the presence of M-protein and prior history of autoimmune disease (Fisher's exact test, p=0.46). Of the 32 patients who underwent cytogenetic studies, 9 (28%) had cytogenetic aberrations involving MALT1. Histological transformation to diffuse large B-cell lymphoma (DLBCL) was confirmed in 3 patients. After a median follow-up time of 53 months (range, 1–142 months), 16 patients had relapsed (MALT lymphoma relapse or transformation to DLBCL), and 1 patient had died due to lymphoma. The 4-year PFS and overall survival rate were 76.3% (95% confidence interval (CI), 60.0–86.7%) and 98.0% (95% CI, 86.6–99.7%), respectively. Among the clinical variables measured at diagnosis, univariate analysis found that advanced stage, three or more extranodal sites, serum soluble IL-2 receptor concentration higher than 700 IU/L, lymphocyte count higher than 1x10^9/l, hemoglobin concentration lower than 12.5 g/dl, and the presence of M-protein adversely affected PFS (p<0.05). In multivariate analysis including these six variables, only the presence of M-protein remained as a poor prognostic factor for PFS (hazard ratio (HR), 14.5; 95% CI, 1.47–142; p=0.021). The 4-year unadjusted PFS for patients with M-protein was 14.8% (95% CI, 7.36–47.6%), which was significantly poorer than that of patients without M-protein (90.9% [95% CI, 74.0–97.0%]; HR, 11.9; 95% CI, 3.46–41.1; log-rank, p<0.001). Seven of the 9 patients who had M-protein received rituximab-containing combination chemotherapy (R-CHOP 5 and R-CVP 2), and 5 patients relapsed in a relatively short time (median, 10 months; range, 2–54 months).
Extranodal MALT lymphoma patients with M-protein had an increased risk of early disease progression. Patients with M-protein were more likely to had advanced stage disease (p=0.005) and more than three extranodal sites (p=0.02) compared to patients without M-protein. However, multivariate analysis showed that the prognostic impact of M-protein was better than that of advanced stage disease and the number of extranodal sites. Further studies are required to determine differences between the biological backgrounds of patients with and without M-protein, and a novel treatment strategy to obtain a durable disease control.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal