Abstract
Anemia is a common symptom in patients with Myelodysplastic Syndromes (MDS) and although erythropoietic agents are often active, it is frequently treated with red blood cell (RBC) transfusions. A substantial proportion of patients might also eventually become transfusion-dependent and, Iron-chelating therapies might be important to minimize complications of iron overload.
To investigate the impact of deferasirox therapy on health-related quality of life (HRQOL) of lower risk transfusion-dependent MDS patients over a one year period. Secondary objectives were to investigate relationships between HRQOL and ferritin levels and to explore the prognostic value of baseline HRQOL on the probability of achieving transfusion independence.
This was a prospective study whose clinical findings (i.e., primary endpoint was safety and tolerability) were previously reported. HRQOL was a secondary endpoint of the study and we herein report, for the first time, HRQOL prospective findings. Eligible patients included: MDS patients 18 years or older, International Prognostic Scoring System (IPSS) low or intermediate-1 risk and diagnosed with transfusional siderosis following a minimum of 20 blood transfusions. Patients received daily oral deferasirox at a dose between 10 and 30 mg/kg of body weight for a period of 1 year. HRQOL was assessed with the EORTC QLQ-C30. HRQOL at baseline and at 3, 6, 9 and 12 months after treatment start. The EORTC QLQ-C30 consists of 30 items and includes five functional scales (physical, role, emotional, social, and cognitive), three symptom (fatigue, nausea and vomiting and pain) and a global health status/QOL scale and six single items (dyspnea, insomnia, appetite loss, constipation, diarrhea and financial difficulties). The mean trend of HRQOL over time was estimated via a linear mixed model with a one-step autoregressive covariance structure. Such covariance structure provided the best model fit among those investigated.
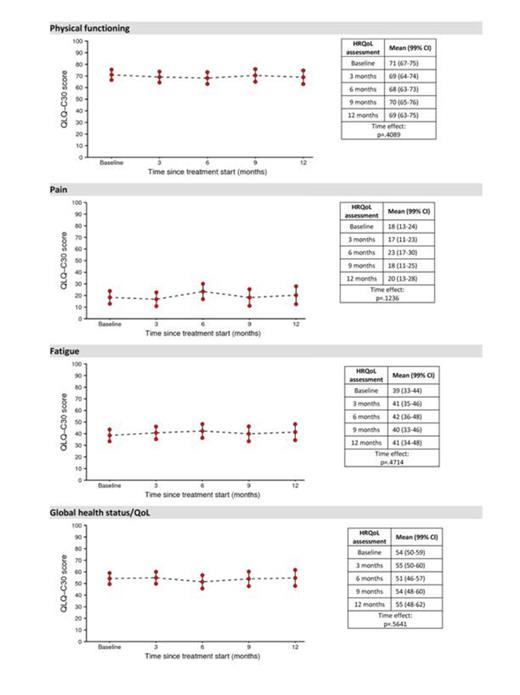
Overall, 159 patients were screened at 37 centers. The median duration of disease at enrollment was 32 months and median number of units of packed RBC received was 37. Seven patients did not start treatment at all and thus there were 152 expected HRQOL forms at baseline assessment. Out of these, 146 patients returned the questionnaire yielding a baseline compliance of 96%. No statistically significant differences over time were found for any scale of the EORTC QLQ-C30. Figure 1 depicts mean scores over time for selected scales of: fatigue, physical functioning, pain and global HRQOL. No HRQOL differences were found between patients with serum ferritin levels lower or higher than 2000 μg/L (pretreatment median value) at baseline. Also, the possible impact of ferritin level on HRQoL over time was estimated via a linear mixed model with a one-step autoregressive covariance structure. Coefficients and p values are reported in table 1. The prognostic impact of baseline HRQOL on the probability of achieving transfusion independence (i.e., defined as freedom from transfusion for 3 consecutive months) was investigated. Higher severity of pain (P=0.007) was associated with a greater likelihood of achieving transfusion independence. Multivariate analysis, controlling for age, IPSS risk score, time from diagnosis, number of previous blood transfusions and baseline ferritin level confirmed the independent value of pain (P=0.003).
Mean HRQoL scores over time
Impact of ferritin level (μg/L) on HRQoL over time.
| EORTC QLQC30 scales . | β . | P . |
|---|---|---|
| Physical functioning | -0.00097 | 0.1444 |
| Role functioning | -0.00164 | 0.0687 |
| Emotional functioning | -0.00084 | 0.1957 |
| Cognitive functioning | -0.00166 | 0.0071 |
| Social functioning | -0.00123 | 0.1385 |
| Global health status/QoL | -0.00057 | 0.4463 |
| Fatigue | 0.000998 | 0.1887 |
| Nausea/Vomiting | 0.000116 | 0.7724 |
| Pain | 0.000383 | 0.6626 |
| Dyspnea | 0.001183 | 0.1891 |
| Insomnia | -0.00056 | 0.5171 |
| Appetite loss | 0.000189 | 0.8143 |
| Constipation | 0.000858 | 0.3019 |
| Diarrhea | 0.000314 | 0.6131 |
| EORTC QLQC30 scales . | β . | P . |
|---|---|---|
| Physical functioning | -0.00097 | 0.1444 |
| Role functioning | -0.00164 | 0.0687 |
| Emotional functioning | -0.00084 | 0.1957 |
| Cognitive functioning | -0.00166 | 0.0071 |
| Social functioning | -0.00123 | 0.1385 |
| Global health status/QoL | -0.00057 | 0.4463 |
| Fatigue | 0.000998 | 0.1887 |
| Nausea/Vomiting | 0.000116 | 0.7724 |
| Pain | 0.000383 | 0.6626 |
| Dyspnea | 0.001183 | 0.1891 |
| Insomnia | -0.00056 | 0.5171 |
| Appetite loss | 0.000189 | 0.8143 |
| Constipation | 0.000858 | 0.3019 |
| Diarrhea | 0.000314 | 0.6131 |
Current findings suggest that Deferasirox therapy does not decrease HRQOL in lower risk transfusion-dependent MDS patients. Patients with higher baseline pain severity seems more likely to achieve transfusion independence and further analysis is needed to understand underlying reasons.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal