Abstract
CLL2007FMP (fit medically patients) is a Randomized Phase-III Trial conducted by the French Cooperative Group on CLL and WM (FCGCLL/WM) and the “Groupe Ouest-Est d'Etude des Leucémies et Autres Maladies du Sang” (GOELAMS), comparing FC plus Rituximab (FCR) to FC plus Campath (FCCam) in previously untreated fit patients with chronic lymphocytic leukemia (CLL). Early results showed that the FCCam regimen was associated with an unfavourable safety profile limiting significantly its use in this indication (Blood 2012). We present here the results of the extended follow up of the CLLFMP2007 trial, with particular emphasis on survival data, minimal residual disease (MRD) and late adverse events.
In this trial, 178 younger (<65) fit patients (pts) (cumulative illness rating scale (CIRS) score of up to 6), were enrolled between November 2007 and January 2009. Cases with del(17p) were excluded. Pts were randomly assigned to receive 6 oral courses of FCR (n=83) or FCCam arm (n=82). The primary endpoint of the study was 3-year progression-free survival (PFS). Secondary endpoints were safety, response to treatment, overall survival (OS) and MRD. MRD evaluation was performed by 6-color flow cytometry in an oligocentric manner. MRD testing was scheduled before therapy initiation and at final evaluation, (i.e. 3 months after completion of immunochemotherapy) where it was to be assessed for all responding patients in both peripheral blood (PB) and bone marrow (BM). Recruitment was interrupted in January 2009 after 165 pts had been randomized due to an excess of mortality in the FCCam arm.
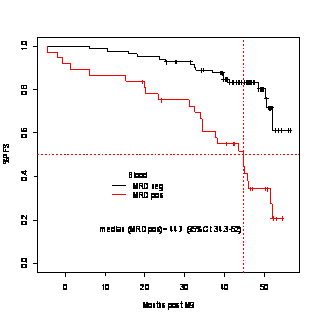
PFS and OS were not significantly different between the two arms. With a median follow-up of 55.5 months (interquartile range, 50-60), 57 pts in the FCCam arm were free of disease progression compared with 50 in the FCR arm, with a 3-year estimated PFS at 81% in both arms (p=0.80). Fourteen pts died in the FCCam arm (7 from progression and 7 from toxicity) and 9 died in the FCR arm (all from progression), with a 3-year estimated survival at 90% vs. 88% (p=0.85). PFS was significantly impacted by IGHV mutational status (p=0.001), Binet stage (p=0.0002) and MRD level. At final evaluation, MRD was established using the result in PB samples (available for 120 patients) and was determined in 103 pts by combining the results from blood and BM samples. Interpretation was based firstly on the use of the classical 10-4 threshold as reference and secondly on the limit of detection of the technique (0.7x10-5). In MRD-positive patients, the median PFS was 44.7 months (PB) whereas it was not reached in the group with MRD lower than 10-4 (p<0.0001, figure 1) ; similar data were found in MRD-positive PB+BM patients with a median PFS of 46 months whereas it was not reached in the group with MRD lower than 10-4 (p=0.002). No significant difference was found regarding OS but follow-up is still short for this evaluation. Similar results were observed when considering the limit of detection of the MRD technique (data not shown). Late toxicities (occurring after the final evaluation at 3 months after the end of treatment or at the ninth month when treatment was prematurely stopped) included : 1 bile duct cancer, 1 myelodysplastic syndrome, 1 transient ischemic attack, 1 lung adenocarcinoma and one prostate cancer in the FCR arm and 3 febrile neutropenia, 3 pneumonia (1 due to legionella), 1 pneumococcal sepsis, 1 bronchitis, 1 toxoplasma eye infection, 1 pyelonephritis, 2 herpes zoster, 1 acrodermatitis, 1 subdural hematoma, 1 autoimmune thrombocytopenia, 1 agranulocytosis, 1 autoimmune haemolytic anaemia in the FCCam arm.
Results of this extended follow-up of the CLL2007FMP trial confirm the absence of superiority of the FCCam regimen on OS and PFS. Interestingly, longer follow-up did not reveal a higher rate of late toxicity in FCCam arm, notably in terms of secondary malignancies; Similarly to early toxicity, late was adverse events were mainly infectious. Finally, MRD status determined by 6-color technique in PB and/or BM at post-treatment evaluation was predictive of PFS whatever the treatment arm. This finding is in line with recent reports in other studies pointing out to the powerful value of MRD as prognostic factor, supporting its use as PFS surrogate primary endpoint in clinical trials.
Feugier:roche: Honoraria. Cazin:roche: meeting invitation Other, Membership on an entity’s Board of Directors or advisory committees; GSK: meeting invitation, meeting invitation Other, Membership on an entity’s Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal