Abstract
SAR650984 (SAR) is a naked humanized IgG1 monoclonal antibody (mAb) that binds selectively to the human surface antigen CD38 highly expressed in multiple myeloma cells and other hematological malignancies. SAR kills tumor cells via 3 different biological mechanisms: Antibody-dependent cellular-mediated cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC) and Induction of apoptosis (pro-apoptosis). Here we present preliminary data from the ongoing first in human, Phase I dose escalation study of SAR in patients with selected CD38+ hematological malignancies. (clinicaltrials.gov: NCT01084252)
The primary objective is to determine the maximum tolerated dose (MTD). Secondary objectives include characterization of safety, pharmacokinetics (PK), pharmacodynamics (PD), immunogenicity and disease response.
SAR is administered as a single agent IV infusion every week (QW) or every 2 weeks (Q2W) to adult patients with selected CD38+ hematological malignancies who have progressed on or after standard therapy or for whom no effective standard therapy exists. An accelerated dose escalation schedule was used for the first 5 dose levels (DL) (0.0001 mg/kg to 0.1 mg/kg Q2W), with one evaluable patient per DL unless DLT was experienced. All subsequent DL (0.3 mg/kg, 1 mg/kg, 3 mg/kg, 5mg/kg, 10 mg/kg, 20 mg/kg Q2W and 10 mg/kg QW), followed the classic 3+3 design for dose escalation based on DLT.
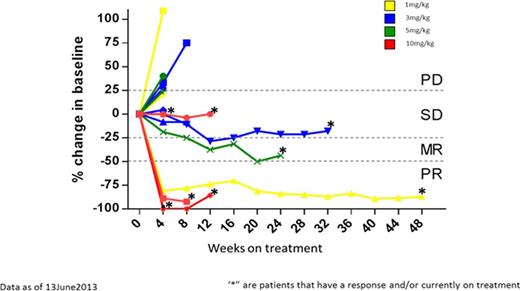
32 patients have been treated across all DLs including 3 patients with NHL, 2 with CLL, and 27 with MM. The 20 mg/kg Q2W and 10 mg/kg QW DLs are currently being evaluated and the MTD has not been reached. DLTs have been limited to Grade (G) 2 infusion reactions during cycle 1 with 1 at DL 0.3 mg/kg and 1 at DL 3.0 mg/kg. This was mitigated by the implementation of routine pretreatment with methylprednisone, diphenhydramine, ranitidine and acetaminophen. The most frequent occurring adverse events (≥ 10%) all DL, regardless of causality, are fatigue (46.9%), nausea (31.3%), pyrexia (28.1%), cough (25%), vomiting (21.9%), hypercalcemia (18.8%), with headache, constipation, bone pain, chills and diarrhea each occurring in 15.6% of patients. In addition, pneumonia, anemia, dysgeusia and hypokalemia each occurred in 12.5% of patients. Serious adverse events considered related to therapy include G 3 pneumonia (6.3%) associated with fever (3.1%), hyperglycemia (3.1%) and one Grade 2 infusion reaction (3.1%). Of the 19 patients treated at DL 1.0 mg/kg to 10 mg/kg Q2W, 1 had CLL, 1 had NHL and 17 had MM. The 17 MM patients were older and heavily pretreated patients; median age of 64 years (range: 55-74); and median of seven prior regimens (range: 2-14). All MM patients had received prior lenalidomide and bortezomib. The median time from diagnosis to first SAR650984 dosing was 6. 8 years (range 1.8 – 16.8 years). Responses in this group (fig 1), according to EBMT MM criteria, included 1 PR at 1 mg/kg (n = 3) and 5 mg/kg (n=3), and 1 MR at DL 3 mg/kg (n = 6). The 10 mg/kg DL demonstrated 3 PR and 2 SD among 6 MM patients treated. For the 19 patients treated at or above the 1 mg/kg DL the median time on treatment is 8 weeks (range 2-50 weeks). Immunogenicity studies show no anti-SAR antibodies. Receptor Occupancy could be detected from DL 1 mg/kg and reached a range of 84.1 to 97.7 % at 10 mg/kg. PK analysis show a more than dose proportional increase of exposure over the 0.03 to 10 mg/kg dose range with clearance in a similar range between 5 mg/kg and 10 mg/kg. No accumulation was observed based on Cmax at cycle 2 over the 0.03 to 3 mg/kg dose range. Tumor growth inhibition threshold was reached at Cmax for 1 patient at DL 5 mg/kg and 5 patients at DL 10 mg/kg.
The safety profile of SAR is predictable and manageable and the MTD has not been reached. SAR demonstrates encouraging single agent activity in patients with heavily pretreated RRMM and warrants further evaluation in this patient population.
Zheng:sanofi: Employment. Daskalakis:sanofi: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal