Abstract
Myelodysplastic syndromes (MDS) comprise a heterogeneous group of myeloid neoplasms characterized by peripheral cytopenia, bone marrow (BM) dysplasia in one or more hematopoietic lineages, and clonal instability with an increased risk to transform to secondary acute myeloid leukemia (AML). In most patients, the erythroid lineage is affected. During the past few years our knowledge about the pathogenesis of MDS, has increased substantially. However, most of these studies focused on CD34+ stem and progenitor cells or granulomonocytic cells, whereas only little is known about genes aberrantly expressed in dysplastic erythroid cells in MDS.
In an attempt to define abnormal gene expression patterns and molecular pathways contributing to erythroid dysplasia in MDS and to identify disease-specific phenotypic and molecular abnormalities in dysplastic erythoid cells, we compared gene expression profiles of erythroid progenitor cells (EryPC) in 10 patients with low-risk MDS (IPSS-R score≤7) and 15 control BM samples. EryPC were defined as CD45low/CD105+ cells and purified from BM samples by multicolor flow cytometry and cell sorting (purity>95%). Gene expression levels were analyzed by Affymetrix array technology (GeneChip U133 Plus 2.0 arrays) and confirmed for a panel of select genes by qPCR. A stringent cut-off (p<0.01; fold-change >3.0) was used to define differences in mRNA expression levels. A total number of 1,180 mRNA species were found to be differently expressed in EryPC in MDS (72 up-regulated, 1,108 down regulated) compared to normal BM EryPC. Among these, a number of genes regulating proliferation and differentiation in lymphohematopoietic cells, including TCEB1 transcription elongation factor B (SIII), the transcriptional repressor YY1, E2F3, and the ATP-dependent RNA helicase DDX5, were found to be downregulated in EryPC in MDS compared to normal BM.
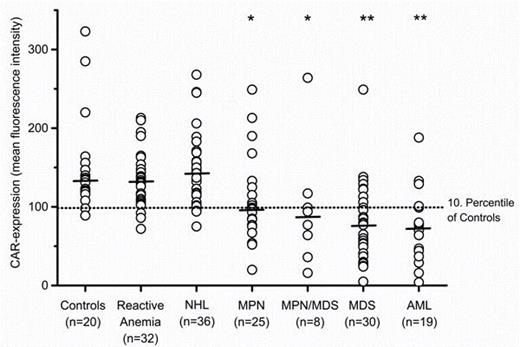
Expression levels of CAR on EryPC determined by flow cytometry. The level of CAR expression on EryPC is depicted as mean fluorescence intensity and indicated by circle in each individual donor. Horizontal lines represent median CAR expression levels in each group of donors. *, p<0.05, and **, p<0.001, compared to healthy controls, as determined by Mann-Whitney U test.
Expression levels of CAR on EryPC determined by flow cytometry. The level of CAR expression on EryPC is depicted as mean fluorescence intensity and indicated by circle in each individual donor. Horizontal lines represent median CAR expression levels in each group of donors. *, p<0.05, and **, p<0.001, compared to healthy controls, as determined by Mann-Whitney U test.
In summary, our data show that the major Coxsackie-Adenovirus Receptor CAR is expressed at abnormally low levels on dysplastic EryPC in a substantial subset of patients with MDS and in most patients with related BM neoplasms. Since CAR expression remains normal in almost all reactive conditions, deficiency states and lymphoid malignancies, abnormal CAR expression may contribute as a potential indicator of (clonal) dysplastic erythropoiesis in myeloid malignancies, including MDS. The biochemical basis of abnormal expression and the possible role CAR may play in the pathogenesis of MDS and in related BM neoplasms, is currently under investigation.
Valent:Novartis: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal