Abstract
Besides a more reliable and frequent measurement of cytogenetic response, it was our aim to find out whether lenalidomide treatment in patients with IPSS low- or intermediate I-risk MDS can foster karyotype evolution (KE) and thus increase the risk of leukemic transformation. Thus, it is an important goal of the Le-Mon-5 study to examine the cytogenetic course under treatment with lenalidomide. In this study, only lower risk MDS patients with an isolated del(5q) are included.
We performed a rigid initial pretreatment screening of bone marrow (bm) aspirates by chromosome banding (CBA) as well as FISH-analysis to ensure an isolated del(5q). For initial screening as well as frequent cytogenetic follow-up every two to three months (FISH analysis of immunomagnetically enriched CD34+ peripheral blood cells (PBC) = CD34+ pb FISH), we used panels of 8 to 13 FISH probes covering the most common aberrations in MDS (Braulke et al. Leuk Res, 2010, 2013). Using this method we intended a reliable surveillance of cytogenetic changes occurring during therapy. Complete cytogenetic remission (CCyR) was defined as: no metaphases with del(5q) and abnormal FISH result below the laboratory threshold (5% EGR1-loss in CD34+ PBC) and partial cytogenetic remission (PCyR) was defined as: >50% reduction of clone size, also including cases with a CCyR at only one time point and those with either only CBA-CCyR or FISH-CCyR.
From the initially screened 145 MDS patients, 84 could be included in the study according to the study inclusion criteria. Currently, follow-up data (at least 2 different time points) are available for 67 patients. An ongoing (>1 time point) CCyR was observed in 22 (33%) patients after a median follow-up of 18 (6-33) months. Thirty-three patients (49%) had a PCyR. Considering only CD34+ pb FISH results 31 (46%) patients showed a complete molecular-cytogenetic remission.
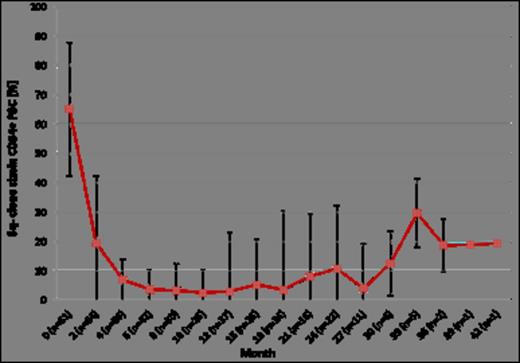
A cytogenetic response was observed after a median of 6 (2-12) months after initiation of therapy for a median duration of 10 (2-25) months. In twelve patients (18%) the size of the del(5q) clone did not change. The median del(5q)-clone size at start of therapy was 60% in responders. After 10 cycles maximum clone size reduction was achieved (n=26) (median: 2.6%; range: 0% - 33.6%), after 24 months of observation clone size in responders (n=22) still was reduced to a median of 10.8% (range: 0.5% -76%) (Figure 1).
Median and standard deviation of del(5q) clone sizes during treatment with lenalidomide
Median and standard deviation of del(5q) clone sizes during treatment with lenalidomide
In 10 of 67 patients (15%) a KE occurred in the del(5q) clone after a median time of 18 months (6-42). This is not increased compared to the rate of spontaneous KE (13%) in a representative MDS cohort (n=729) studied recently by our group by banding analysis (Cevik et al. DGHO 2013, accepted for oral presentation). Remarkably, in one patient a KE with loss of a p53 allele and a 20q- deletion resolved after the 8th cycle of lenalidomide and after the 10th cycle a complete cytogenetic remission was achieved (Figure 2). In two additional cases, the del(5q) clone was completely eliminated, however a new cell clone with independent abnormalities (t(3;3)(q21;q26), second case: inv(11)(p15q23)del(11)(q13q22)) emerged under lenalidomide. There is a growing body of evidence that these clones were preexistent initially but below the cytogenetic detection level and were possibly suppressed by the del(5q) clone until its cytogenetic remission.
Cytogenetic course in a patient with transient KE
Our interim results show that:
A cytogenetic response to lenalidomide can be achieved in >80% of pts. with isolated del(5q) and lower-risk MDS
CBA can be complemented reasonable by CD34+ pB FISH, which allows a reliable monitoring of response and KE
Cytogenetic response to lenalidomide is durable (median 10 months as yet)
KE is not increased in this patient group under treatment with lenalidomide
In one case with KE and acquisition of additional p53 allelic loss and 20q-deletion continuation of lenalidomide finally lead to a CCyR
Platzbecker:Celgene: Honoraria. Nolte:Celgene: Honoraria, Research Funding. Giagounidis:Celgene: Honoraria. Götze:Celgene Corp: Honoraria. Schlenk:Celgene: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Chugai: Research Funding; Amgen: Research Funding; Novartis: Research Funding; Ambit: Honoraria. Bug:Celgene: Honoraria, Research Funding. Germing:Celgene: Honoraria. Haase:Celgene: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal