Abstract
Subcutaneous (SC) omacetaxine mepesuccinate (OMA) is indicated for the treatment of CP and AP CML in adults with resistance/intolerance (R/I) to ≥2 tyrosine kinase inhibitors (TKIs). Unlike TKIs, OMA inhibits protein synthesis and is not a direct inhibitor of Bcr-Abl kinase activity.
The clinical activity of OMA was demonstrated in a combined cohort of patients from 2 single-arm trials. The cohort consisted of patients who had received ≥2 approved TKIs and, at a minimum, documented evidence of R/I to dasatinib and/or nilotinib. This is the final ≥24-month follow-up analysis in a cohort subset from the 2 studies as originally approved by the FDA.
Patients received OMA 1.25 mg/m2 bid SC in 28-day cycles: 14 days for induction and 7 days as maintenance, adjusted for tolerability. Primary endpoints were major cytogenetic response (MCyR) for CP and major hematologic response (MaHR) for AP. Secondary objectives included time to onset and duration of response, progression-free survival (PFS), overall survival (OS), and adverse events (AEs). Survival data were collected after study discontinuation. Efficacy was analyzed by a data monitoring committee (DMC) on-study and investigators during follow-up for the cohort used for approval of OMA, which excluded patients with a best response at baseline or from a site without verified protocol compliance. Safety was analyzed for all CP and AP patients receiving SC OMA.
As of October 12, 2012, the efficacy analysis cohort included 76 CP and 35 AP patients. The safety group included 163 patients (108 CP and 51 AP, plus 4 AP patients from a prior study). The majority of the 76 CP (median age, 59 y; range, 26-83 y) and 35 AP patients (median age, 64 y; range, 23-83 y) had received hydroxyurea (CP 41/76, AP 22/35) and 3 prior TKIs (CP 36/76, AP 22/35). At data cutoff, 5 CP patients and 1 AP patient continued OMA treatment (CP: 57, 43, 57, 52, and 48 months; AP: 52 months).
Median treatment duration was 7.5 (range, 0-55.6) months for CP and 1.6 (0-49.7) months for AP patients. Median total months of follow-up for survival was 29.5 (95% CI, 17.6-36.4) for CP and 14.3 (95% CI, 4.7-18.7) for AP patients. Of patients receiving ≥2 cycles, 53/60 CP and 16/26 AP patients had a cycle delay; median number of cycles requiring delay per patient were 4 (CP) and 1 (AP).
DMC-assessed responses were unchanged at 24 months; only investigator assessments were performed during follow-up. The MCyR rate for CP was 18% (14/76) with a mean time to onset of 3.5 months and median duration of 12.5 months. For AP, the MaHR rate was 14% (5/35) with a mean time to onset of 2.3 months and median duration of 4.7 months. No AP patients achieved MCyR. For ongoing patients, responses at data cutoff were complete cytogenetic response for all CP patients and hematologic improvement for the AP patient.
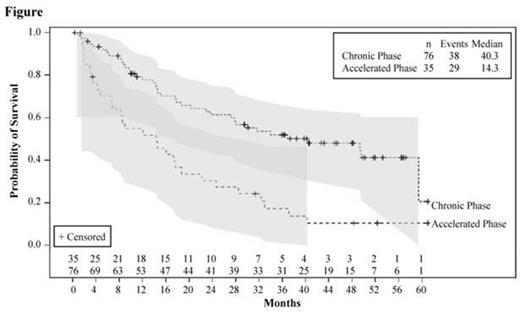
Median PFS was 9.6 months for CP and 3.6 months for AP patients. At data cutoff, 16 CP and 1 AP patients had not progressed. Survival data were collected after therapy discontinuation; however, of the 6 patients in the efficacy cohort who remained on treatment at data cutoff, 2 (both CP) lost response as assessed by investigators. Based on the 24-month update, median OS was extended from 33.9 to 40.3 (95% CI, 23.8 to not reached) months for CP patients and was unchanged for AP patients at 14.3 (95% CI, 6.7-18.7) months (Figure).
Safety and tolerability across the 24-month analysis were unchanged from the original analysis. The most common AEs (≥20%) were thrombocytopenia, anemia, neutropenia, diarrhea, nausea, fatigue, asthenia, and pyrexia for CP and AP patients; leukopenia and headache were ≥20% for CP only; febrile neutropenia was ≥20% for AP only. Patients with severe myelosuppression were at risk for infections and hemorrhage. Grade 3/4 hematologic laboratory toxicities for CP and AP patients were thrombocytopenia (CP 92/108, AP 44/50), neutropenia (CP 88/108, AP 35/50), leukopenia (CP 82/108, AP 30/50), and anemia (CP 65/108, AP 39/50). There were 53 deaths in the CP and 38 in the AP groups in the ≥24-month period; the most common causes were disease progression (25, 15), unknown (13, 17), sepsis (5, 0), and hemorrhage (cerebral [2, 3], pulmonary [1, 1]).
This 24-month update demonstrates that OMA induces clinically meaningful and durable responses in a subset of heavily pretreated patients with CP or AP CML who failed prior TKI therapies. Most grade 3/4 AEs were related to myelosuppression.
Support: Teva BPP R&D, Inc.
Cortes:BMS : Research Funding; Novartis: Research Funding; Pfizer: Consultancy, Research Funding; Teva: Consultancy, Research Funding; Ariad: Consultancy, Research Funding. Wetzler:Teva: Honoraria, Membership on an entity’s Board of Directors or advisory committees. Lipton:Ariad: Consultancy, Equity Ownership, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Teva: Consultancy, Honoraria, Research Funding. Khoury:Teva: Honoraria. Michallet:Astellas: Honoraria; Merck: Honoraria; Genzyme: Honoraria, Membership on an entity’s Board of Directors or advisory committees; Janssen-Cilag: Honoraria, Membership on an entity’s Board of Directors or advisory committees; Novartis: Research Funding; Teva: Membership on an entity’s Board of Directors or advisory committees; Pfizer: Honoraria; BMS: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding. Baccarani:BMS: Consultancy, Honoraria; Ariad: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Teva: Consultancy; Novartis: Consultancy, Honoraria. Rea:BMS: Honoraria; Teva: Honoraria; Novartis: Honoraria. Chuah:BMS: Honoraria; Novartis: Honoraria. Parikh:Roche: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding; Dr Reddys: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding; Boehringer Ingelheim: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding; Glenmark: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding; Eisai: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding; Spectrum Pharmaceuticals: Equity Ownership; OncoRx: Equity Ownership; Biocon: Research Funding; GSK: Research Funding; Intas: Research Funding; Alkem: Research Funding. Li:PharmaStat, LLC: Consultancy. Munteanu:Teva: Employment, Equity Ownership. Brown:Teva: Employment, Equity Ownership. Nicolini:Novartis: Consultancy, Honoraria, Research Funding; BMS: Honoraria; Teva: Honoraria; Ariad Pharmaceuticals: Honoraria; Pfizer: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal