Abstract
Currently there is no standard for second-line salvage therapy in patients with acute myeloid leukemia (AML) who do not respond to initial induction therapy. The efficacy of current recommended second-line salvage regimens seems to be similar, but the tolerability can vary significantly. Anecdotally, high-dose cytarabine (HiDAC) monotherapy seems to be better tolerated than other recommended combination salvage regimens. Therefore, we conducted a retrospective analysis to evaluate the MSKCC experience with HiDAC monotherapy as compared to combination with other chemotherapeutic agents.
We included AML patients ≥ 18 years of age who did not respond to first induction therapy and received HiDAC (group 1; cytarabine 3g/m2 q12h for up to 12 doses) or intermediate- or high-dose cytarabine (1g/m2 or 3g/m2 at various numbers of doses) in combination with other agents (group 2) as second-line salvage, between the years 2003 and 2013. The combination group (group 2) consisted of regimens such as: MEC, CLAG, FLAG-I, and mitoxantrone or idarubicin with HiDAC. We evaluated the overall response rate (ORR), relapse free survival (RFS), and overall survival (OS). Univariate analysis was used to evaluate the potential association between outcomes and the following patient characteristics at the time of diagnosis: peripheral and marrow blast count, white blood cell count (WBC), platelet count, anthracycline agent used in first induction, cytogenetics risk, age, comorbidities (cardiac history, diabetes, hypertension, pulmonary, renal, and hepatic compromise), and AML subtype such as de novo (DN), antecedent (AHD), and treatment-related (t-AML).
Group 1 (monotherapy) had 30 patients and group 2 (combination) had 77 patients. Patient characteristics were similar between the two groups with the exception of age, anthracycline type (daunorubicin vs idarubicin) used in first induction, and transplant status. Group 1 had more patients younger than 58 years old (median), more daunorubicin use, and more patients who underwent allogeneic stem cell transplantation. The side effects in group 1 were notable for pneumonia, C. diff, and bacteremia. The most notable side effects in group 2 were pneumonia, C. diff, bacteremia, sepsis, and treatment-related deaths. No statistically significant difference was observed in ORR (36.7% vs 33.3%; p = 0.922), RFS (9.3 vs 5.9 months; p = 0.53), and OS (12.9 vs 6.8 months; p = 0.329) between group 1 and group 2 respectively.
Univariate screening showed no difference in ORR with respect to patient characteristics in group 1. In group 2, ORRs with regard to AML subtype were 64%, 28%, 8% for DN, AHD, t-AML respectively (p = 0.014), and a higher ORR was observed in patients with WBC ≥ 10 K/mcL (58.3% vs 41.7%; p = 0.051) and patients with good or intermediate cytogenetic risks (64% vs 36%; p = 0.017). For RFS, patients in group 1 with WBC > 10 K/mcL were associated with 3 times higher risk of relapse or death (p = 0.029). For RFS in group 2, patients with AHD and t-AML were associated with higher risk of relapse (HR (95% CI): 1.3 and 3.7 respectively, p = 0.002) compared to DN. For OS, patients in group 1 with WBC > 10 K/mcL were at 2.7 times higher risk of death (p = 0.049), an association which was not present in group 2. For OS, patients in group 2 with t-AML and AHD were at 4.2 times and 1.7 times higher risk of death than DN (p = 0.001) and this difference was not present in group 1. Patients with poor cytogenetic risks or comorbidities of interest did worse in terms of overall RFS (p < 0.001, p = 0.02 respectively) and OS (p < 0.001, p = 0.009 respectively). Outcomes did not seem to be associated with type of anthracycline used in first induction or platelet count at diagnosis.
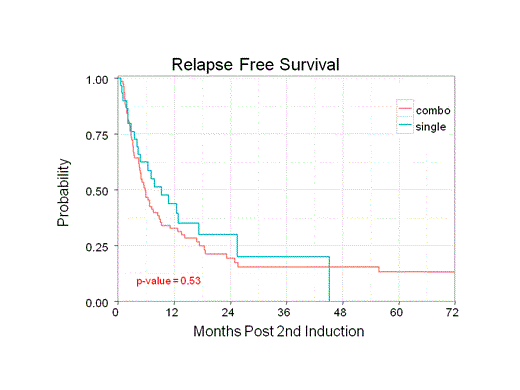
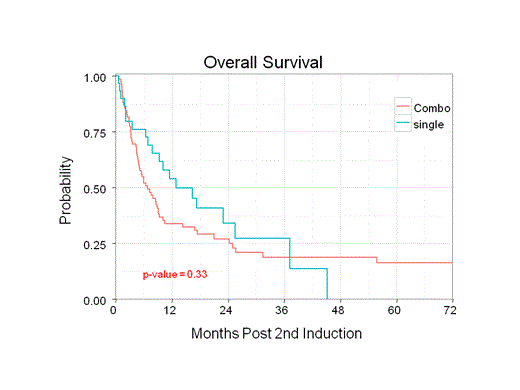
Recognizing the limitations of a retrospective study, our analysis showed no difference in ORR, RFS, and OS for HiDAC monotherapy compared to intermediate- or high-dose cytarabine combinations. Our univariate analysis suggests statistically significant differences in ORR, RFS, and OS with regard to AML subtype, WBC at time of diagnosis, comorbidities, and cytogenetics risk. We are subcategorizing the combination group into intermediate-dose and high-dose cytarabine combination and compare them to monotherapy. We are looking at additional molecular genetics information besides NPM1, CEBPA, and FLT3 that could also influence outcomes.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal