Abstract
CCAAT/enhancer binding protein alpha (CEBPα) is one of the crucial transcription factors involved in hematopoietic differentiation and leukemogenesis. CEBPα promotes myeloid differentiation by up-regulation of lineage specific genes and by cell proliferation arrest. Epigenetic regulation of CEBPα expression through DNA methylation has been demonstrated in acute myeloid leukemia (AML) (Figueroa et al, Cancer Cell, 2010). However, only limited data are available regarding CEBPA promoter methylation and its expression in B cell precursor acute lymphoblastic leukemia (BCP-ALL).
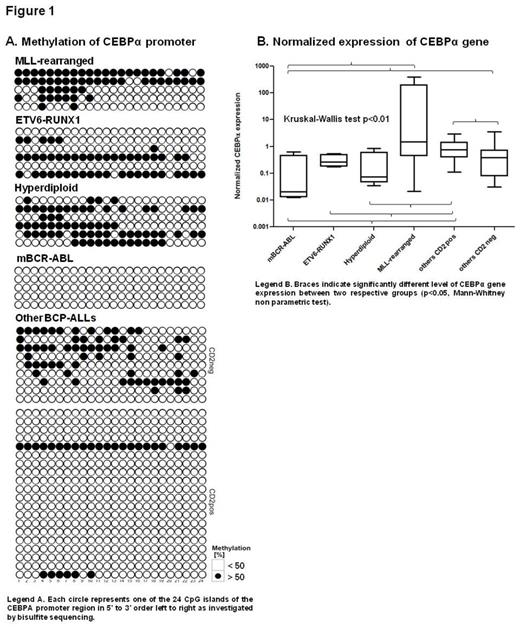
In previous study we found association of CD2 (LFA-2) aberrant expression and switch to the monocytic lineage during the early phase of treatment in BCP-ALLs (Slamova et al, ASH 2012). We were interested if a possible link between hypomethylation of CEBPA promoter correlates with aberrant expression of CD2. There was a significant association between aberrant expression of CD2 antigen and hypomethylation in CEBPA promoter in BCP-others (Fisher exact test, p<0.0001). Interestingly, in the only hypomethylated ETV6-RUNX1pos case we found aberrant CD2 expression on blasts, which is exceptional in ETV6-RUNX1pos ALL.
We next asked whether methylation of CEBPA promoter correlates with CEBPα expression. It is generally accepted that promoter hypomethylation is often associated with increased expression of the relevant gene. Our data prove that in general, this holds true also for BCP-ALL. However, in two genetically defined subsets we observed either high expression despite hypermethylation (MLL-rearranged ALL) or low expression despite hypomethylation (mBCR-ABLpos ALL) (Figure 1B). In BCP-others hypomethylation of CEBPA promoter was significantly associated with upregulation of myeloid antigens (CD14 and/or CD33) and downregulation of B cell marker CD19 on blasts during the first weeks of the treatment (Fisher test, p=0.0009).
Methylation status of CEBPA promoter correlates with genetic subtypes of BCP-ALL. The notion that hypomethylation leads to overexpression was confirmed in majority of BCP-ALLs, while in mBCR-ABLpos and MLL gene rearranged BCP-ALL it did not follow this pattern. Hypomethylation of CEBPA promoter in BCP- others correlates with CD2 expression on blasts and increased CEBPα gene expression. During the early phase of the treatment in other BCP-ALLs with hypomethylated CEBPA promoter increase of myeloid and decrease of B lymphoid markers on blasts was observed.
Supported by: GACR P301/10/1877, GACR P304/12/2214, GAUK 914613, UNCE 204012, NT13462, NT12397- 4, project for conceptual development of research organization (Ministry of Health, CZ) 00064203, the FACS Aria instrument was supported by EU-Prague project CZ.2.16/3.1.00/24022
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal