Abstract
Lenalidomide is an immunomodulatory drug with effects on the innate immune system that may enhance antibody-dependent cell mediated cytotoxicity and synergize with the action of rituximab. Several studies have shown that Fc-gamma receptor RIIIA (FCGR3A) polymorphisms at amino acid 158 (V/V vs. V/F and F/F) have a significant impact on overall response rate, complete response rate, and time to progression in lymphoma patients (pts) receiving single-agent rituximab (Cartron 2002, Weng 2003). For example, the objective response rates at one year post treatment with rituximab were 75% in the FCGR3A 158 V/V group, as opposed to 26% in FCGR3A 158 V/F or F/F group (FCGR3A-F carriers with at least one F allele) with 2-year progression-free survival (PFS) of 14% for FCGR3A-F carriers (Weng 2003). To test the efficacy of lenalidomide combined with rituximab, we conducted a single center, open label phase II clinical trial in pts with indolent B-cell or mantle cell lymphomas previously unresponsive to rituximab. We report here the efficacy of the combination of lenalidomide and rituximab in FCGR3A-F carriers refractory to rituximab.
Eligible pts had relapsed/refractory indolent B-cell or mantle cell lymphoma with measurable disease that had either failed to respond to or progressed within six months of a standard course of rituximab monotherapy (375 mg/m2 weekly for at least four weeks) or a prior rituximab-containing chemotherapy regimen. All pts in this analysis of FCGR3A-F carriers had failed to respond to a prior rituximab or rituximab-containing chemotherapy regimen. In Part I (lenalidomide + dexamethasone), pts received two 28-day treatment cycles of lenalidomide 10 mg every day and dexamethasone 8 mg once weekly. After assessment of response to Part I, all pts received four weekly doses of rituximab 375 mg/m2 during cycle 3 (Part II: lenalidomide + dexamethasone + rituximab). Per protocol, pts received only a single four week course of rituximab during cycle 3. Lenalidomide + dexamethasone were continued during and subsequent to rituximab; stable and responding pts continued lenalidomide + dexamethasone until disease progression or development of clinically unacceptable toxicity. Response assessment after Part II was performed three months after the first dose of rituximab.
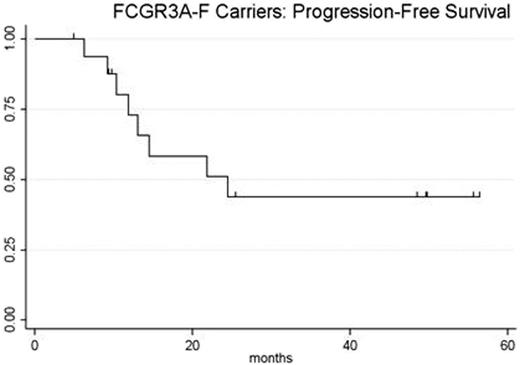
Eighteen pts had FCGR3A genotying performed; 1 patient had the FCGR3A 158 V/V genotype and 17 pts were FCGR3A 158-F carriers. Of the 17 FCGR3A-F carriers, diagnoses included follicular (n = 12), mantle cell (n = 2), small lymphocytic (n = 2), and marginal zone (n = 1) lymphomas; median age was 59 years (range: 35 - 73); male: female ratio was 7:10; median number of prior therapies was 3 (range: 1 - 6). For all FCGR3A-F carriers, overall response rate (ORR) after Part I was 24% (3 CR; 1 PR; 12 SD; 1 PD) and ORR after Part II was 53% (5 CR; 4 PR; 7 SD; 1 PD) [Figure 1]. For 12 pts with follicular lymphoma who were FCGR3A-F carriers, ORR after Part I was 25% (2 CR; 1 PR; 9 SD) and ORR after Part II was 50% (4 CR; 2 PR; 5 SD; 1 PD). At 12 months after receiving rituximab (month 14), ORR for FCGR3A-F carriers was unchanged (50%). Median PFS is 24.5 months (95%CI 18 - 67) with median follow-up of 52 months (range: 11 - 60 months) [Figure 2]. Grade 3 or 4 non-hematologic toxicities in FCGR3A-F carriers included hypokalemia (2/17 pts), hypophosphatemia (2/17 pts), hyperuricemia (1/17 pts), pulmonary embolism (1/17 pts), pneumonia (1/17 pts), diarrhea (1/17 pts), transaminitis (1/17 pts), and tumor flare (1/17 pts).
In FCGR3A-F carriers with indolent B-cell or mantle cell lymphomas refractory to rituximab, the combination of continuous daily lenalidomide, low-dose weekly dexamethasone, and a single four week course of rituximab during cycle 3 achieves a high overall response rate with relatively durable responses. Compared to the 12 month ORR and 2 year PFS for FCGR3A-F carriers previously reported in predominantly relapsed/refractory follicular lymphoma patients (Weng 2003), our results compare favorably (ORR 50% vs 26%, PFS 50% v 14%). The improvement in response during lenalidomide + low-dose dexamethasone following rituximab suggests that this combination overcomes the unfavorable impact of the FCGR3A-F polymorphism on rituximab responsiveness.
Off Label Use: therapy of non-Hodgkin lymphoma with lenalidomide. Schuster:Celgene Corp: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal