Abstract
Paroxysmal nocturnal hemoglobinuria (PNH) often affects young adults, including women of childbearing age. Because PNH hemolysis increases the risk of complications such as thrombosis during pregnancy and the postpartum period, proper management should be provided for pregnant patients with PNH. Eculizumab, a C5 complement inhibitor, could be a therapeutic option for such patients.
Japan PNH Study Group aimed to survey the current status of management of pregnant PNH patients and to propose a consensus recommendation for such a challenging situation in the treatment of PNH. Clinical data were collected from 10 pregnant PNH patients from 7 institutes and analyzed. We had consensus meetings 5 times since January 2012.
Summary of patients' characteristics are shown in Table. Age of the patients at the time of conception ranged from 29 to 40 years old (median 30). Plasma levels of LDH at the baseline assessment were 200-2300 IU/L (median 1900). PNH clone sizes in granulocytes were 1.6-96.0% (median 81). Prophylactic anticoagulation with heparin was employed in all patients except one who showed little hemolysis due to a small size of PNH clone. Four patients received eculizumab during pregnancy with favorable efficacy. Complications including preeclampsia, hemolytic episodes and deep venous thrombosis were manageable. Newborn babies were all healthy. A negligible amount of eculizumab was detected in cord blood of three babies.
Summary of patients' characteristics
| UPN | Age (Yrs) | Delivery method | Eculizumab use | Complication | Outcome of baby | Anti-coagulation |
| A-1 | 34 | Vaginal | All period | Breakthrough hemolysis | Healthy | UFH |
| B-1 | 29 | Vaginal | From 19 wks | No | Healthy | UFH |
| C-1 | 30 | C-section | From 27 wks | Preeclampsia | Premature, but healthy | LWMH |
| D-1 | 37 | C-section | All period | No | Healthy | LWMH |
| E-1 | 30 | Vaginal | No | No | Healthy | No |
| E-2-1 | 30 | Vaginal | No | No | Healthy | UFH |
| E-2-2 | 34 | Vaginal | No | No | Healthy | UFH |
| F-1 | 30 | C-section | No | Hemolytic attack | Healthy | LWMH |
| F-2 | 41 | C-section | No | DVT | Healthy | UFH |
| G-1 | 30 | C-section | No | No | Healthy | UFH |
| UPN | Age (Yrs) | Delivery method | Eculizumab use | Complication | Outcome of baby | Anti-coagulation |
| A-1 | 34 | Vaginal | All period | Breakthrough hemolysis | Healthy | UFH |
| B-1 | 29 | Vaginal | From 19 wks | No | Healthy | UFH |
| C-1 | 30 | C-section | From 27 wks | Preeclampsia | Premature, but healthy | LWMH |
| D-1 | 37 | C-section | All period | No | Healthy | LWMH |
| E-1 | 30 | Vaginal | No | No | Healthy | No |
| E-2-1 | 30 | Vaginal | No | No | Healthy | UFH |
| E-2-2 | 34 | Vaginal | No | No | Healthy | UFH |
| F-1 | 30 | C-section | No | Hemolytic attack | Healthy | LWMH |
| F-2 | 41 | C-section | No | DVT | Healthy | UFH |
| G-1 | 30 | C-section | No | No | Healthy | UFH |
Recently successful pregnancy outcomes have been reported in patients with PNH on eculizumab therapy during pregnancy, and we also experienced additional 4 cases. As eculizumab inhibits intravascular hemolysis itself, it can be expected to lower the risk of complications during pregnancy and postpartum period in women with PNH. Eculizumab is a hybrid of IgG4 and IgG2, and the latter is known to be less prone to cross the placenta. In fact eculizumab concentrations in the cord blood was much lower than therapeutic levels. Importance of anticoagulation therapy has been emphasized for the management of pregnancy with PNH. Theoretically, if intravascular hemolysis is completely inhibited by eculizumab, the risk of thromboembolic complication would become as low as that in non-PNH pregnancies. It is not clear whether the use of anticoagulant is mandatory for the pregnant patients who are receiving eculizumab, although the use of anticoagulant should not be hesitated.
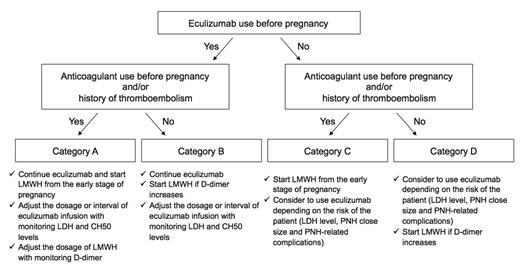
Eculizumab could be safely and effectively used in pregnant patients with PNH. Taking these findings into account, we propose the following protocol for the management of a pregnancy with PNH, as shown in Figure.
Figure. A proposed protocol for the management of a pregnancy in PNH.
Figure. A proposed protocol for the management of a pregnancy in PNH.
The patients are classified into 4 categories based on eculizumab use and the history of venous thromboembolisms or anticoagulant use.
Usuki:Alexion Pharmaceuticals: Speakers Bureau. Urabe:Alexion Pharmaceuticals: Speakers Bureau. Kawaguchi:Alexion Pharmaceuticals: Honoraria. Miura:Alexion Pharmaceuticals: Research Funding. Morita:Alexion Pharmaceuticals: Research Funding. Nishiwaki:Alexion Pharmaceuticals: Research Funding. Ninomiya:Alexion Pharmaceuticals: Honoraria. Gotoh:Alexion Pharmaceuticals: Honoraria. Shichishima:Alexion Pharmaceuticals: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding. Nishimura:Alexion Pharma: Research Funding, Speakers Bureau. Kanakura:Alexion Pharmaceuticals: Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal