Abstract
In primary hemostasis, platelets adhere, activate, and aggregate at the wall of an injured vessel to form a hemostatic plug for the cessation of bleeding. After activation, platelets generate myosin-driven contractile forces to compact the size of the plug in order to reduce the space between platelets and prevent their disaggregation. Hemodynamic shear can be a major effector of platelet function in hemostasis, but its effect on the ability of platelets to produce contractile forces is an open question. Studying the dynamics of platelet aggregation and platelet force generation under hemodynamic shear can provide important insights into hemostasis and thrombosis.
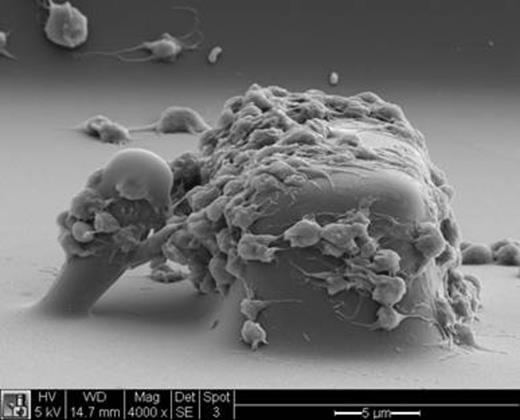
We have developed a microfluidic device that uses microscale blocks to induce platelet aggregation and microscale posts to measure platelet forces in a hemostatic plug. Whole human blood in heparin or citrate is pumped through a microfabricated chip containing microchannels with arrays of blocks and posts arranged along the bottom of a microchannel (Fig. 1). The surface of the blocks and posts are pre-coated with von Willebrand factor and type I collagen to allow for platelet adhesion. As blood is passes over a block, its rectangular shape induces a high shear rate that causes platelets to aggregate on its surface. A flexible micropost is situated behind each block. As platelets aggregate between the block and post, their contractile forces causes the post to bend toward the block. The deflection of the post is recorded under fluorescence microscopy and analyzed using quantitative image analysis of the videos. Since a microscale post bends like a cantilever beam, its deflection can be used to quantify the forces of platelets.
Blebbistatin, a myosin inhibitor, was used to confirm that deflection of the posts by the platelets in heparinized blood was due to myosin activity. When blood was incubated with 2-MeSAMP, a P2Y12 antagonist, platelets were able to aggregate, but their ability to generate contractile forces was substantially reduced. This finding indicates that ADP activation is needed for platelet contractility under shear. The rate of hemodynamic shear was found to influence platelet function, for the rate of platelet aggregation and force generation were found to increase for blood sheared from 2000 to 12,000 s-1. Moreover, platelet aggregation and contractile forces were reduced when glycoprotein Ib-V-IX complex and integrin αIIbβ3 were inhibited with antibody AK2 and antibody fragment c7E3 Fab, respectively. When citrated blood was incubated with tissue plasminogen activator, platelets aggregate and produced contractile forces that increased steadily within the first ten minutes, but then the forces began to subside.
Our device can be used to study the role of hemodynamic shear in platelet function and gives insights into the role of platelet forces during hemostasis. Its microscale dimensions also allow us the study the biomechanics involved in the formation of a hemostatic plug during its early stages of growth and stability.
Platelet forces are measured using blocks and posts in a microfluidic device. As platelets in blood passes around the block, they experience a high shear rate that causes their adhesion, aggregation, and generation of force that bends the post towards the block.
Platelet forces are measured using blocks and posts in a microfluidic device. As platelets in blood passes around the block, they experience a high shear rate that causes their adhesion, aggregation, and generation of force that bends the post towards the block.
White:Vidacare Corp: Honoraria; Stasys Medical Corp: Consultancy, Equity Ownership, Membership on an entity’s Board of Directors or advisory committees, Patents & Royalties; NIH: Research Funding; Coulter Foundation: Research Funding; Washington State Life Sciences Discovery Fund: Research Funding. Sniadecki:Stasys Medical Corporation: Equity Ownership, Founder Other, Membership on an entity’s Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal