Abstract
Monotherapy with clinically available chelators, namely deferoxaime (DFO), deferasirox (DFX) or deferiprone (DFP) is effective but often slow and suboptimal. Combinations of DFO with DFP have been used clinically to enhance cellular iron mobilization but the conditions under which this occurs have not been studied systematically. With the emergence of DFX, the possibility exists to combine this with either DFO or DFP to enhance chelation. We have developed a system to study the optimal concentrations and times of exposure to these chelators, alone or in combination for maximising cellular iron removal. Isobol modeling has been used to determine whether interaction is additive or synergistic. The demonstration of synergy would imply the primary chelator acting as a ‘sink’ for iron chelated and donated to this sink by low concentrations of a secondary ‘shuttle’ chelator as shown in plasma (Evans et al. TransL. Res, 2010).
Human hepatocellular carcinoma (HuH-7) cells were chosen as hepatocytes are the major cell of iron storage in iron overload. Iron concentration was determined using the ferRozine (Riemer et al. Anal Biochem. 2004). A threefold increase of intracellular iron compared to control was obtained by serially treating cells with 10% FBS RPMI media. The cells were then exposed to iron chelator then lysed and intracellular iron concentration determined via the ferrozine assay, normalized against protein content. Cell viability was assessed using 0.4% Trypan blue as well as Acridine Orange /Propidium Iodide and was consistently > 98%. Isobolograms were constructed (Tallarida et al, Pharmacol Ther, 2010) as well as a the synergy index (QUOTE 1-1/R) x 100 (%), where R = difference of areas between the line of additivity and the curve of synergy on the isobologram. This index represents how much of the obtained effect exceeds that expected by additivity of two chelators.
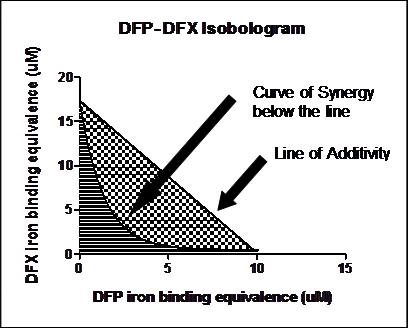
Monotherapy with DFP, DFX or DFO at clinically relevant concentrations of 1 to 30µM iron binding equivalents (IBE), induced both dose and time dependent cellular iron removal. Dual therapy combinations of all 3 chelators enhanced iron removal at 4, 8 and 12 hours. At 4 hours of incubation, whereas 10µM DFO alone had no demonstrable effect on cellular iron removal, addition of DFP at as little as 1µM IBE increased cellular iron removal. Table 1 shows examples of cellular iron removal at specimen chelator concentrations alone or in combination at 8h. The combination of DFX with DFO, DFX with DFP and DFP with DFO all resulted in enhanced cellular iron removal. The combination of DFP and DFX was the most effective. Isobol plot analysis from multiple chelator concentrations demonstrated synergy for all pairs at 4 and 8 hours of exposure. The derived synergy index at 8h indicates that when DFX and DFO are combined, 49% of the chelation effect is due to synergy in this system and 51% in the case of DFP and DFO combination. Most interestingly, the synergistic effect is even greater, in the case of the two oral chelators DFP and DFX when in combination (59%). Figure 1.
Examples of % total cellular iron removal at 8h with monotherapy and combination treatments
| Chelators | (%) Iron Removal | Chelators | (%) Iron Removal | Chelators | (%) Iron Removal |
| DFO 10µM | 30.7 | DFX 10µM | 25.6 | DF10µM | 27.5 |
| DFO 10µM + DFX 5µM | 38.2 | DFX 10µM +DFO 5µM | 36.9 | DF10µM +DFO 5µM | 38.3 |
| DFO 10µM + DFP 5µM | 39.3 | DFX 10µM + DFP 5µM | 41.7 | DF10µM +DFX 5µM | 43.2 |
| Chelators | (%) Iron Removal | Chelators | (%) Iron Removal | Chelators | (%) Iron Removal |
| DFO 10µM | 30.7 | DFX 10µM | 25.6 | DF10µM | 27.5 |
| DFO 10µM + DFX 5µM | 38.2 | DFX 10µM +DFO 5µM | 36.9 | DF10µM +DFO 5µM | 38.3 |
| DFO 10µM + DFP 5µM | 39.3 | DFX 10µM + DFP 5µM | 41.7 | DF10µM +DFX 5µM | 43.2 |
Remarkably low concentrations of a second chelator are required to enhance cellular iron removal by the primary chelator. Isobol analysis shows synergy rather than additivity as the mechanism for enhanced chelation for all 3 combinations, implying a ‘shuttle’ and ‘sink’ effect. Interestingly, the combination of two oral chelators DFP and DFX showed the most marked enhancement of cellular iron removal, without cellular toxicity, suggesting a potentially powerful therapeutic approach, provided this is also well tolerated clinically. The long plasma half life of once daily oral DFX will allow a continuous ‘sink’ for iron shuttled by the shorter acting DFP.
Line of Additivity
Curve of Synergy below the line
Figure 1. Isobologram demonstrating the synergistic action of DFP and DFX
Figure 1. Isobologram demonstrating the synergistic action of DFP and DFX
Porter:Novartis: Consultancy, Honoraria, Research Funding; Shire: Consultancy, Honoraria; Celgene: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal