Abstract
High dose chemotherapy and autologous stem cell transplantation (ASCT) has become part of the standard of care for patients with multiple myeloma (MM). However, this process is often associated with a high symptom burden, particularly for older patients and those with concurrent amyloidosis. We have previously shown that symptom burden during ASCT reaches a peak at the time of WBC nadir and is associated with elevated serum levels of IL-6. In addition, other studies have suggested a dose-response relationship between CD34+ cells dose and rate of neutrophil recovery during ASCT. We therefore hypothesized that higher doses of CD34+ stem cells would be associated with an improved symptom outcome, not only due to a potentially shorter time to engraftment, but also through cytokine modulation.
We conducted a prospective, randomized controlled trial of patients undergoing ASCT for MM (age > 60) or AL amyloidosis. Patients were randomized to receive either a standard (4-6 e6 CD34+ cells/kg) or high (10-15 e6 CD34+ cells/kg) dose of stem cells after high dose melphalan (200 mg /m2). Symptom burden was assessed at baseline and multiple time points throughout the ASCT process via the MD Anderson Symptom Inventory (MDASI), a validated tool for assessing cancer-related symptom burden. Symptoms were scored on a scale of 1-10 with 10 being the most severe. The primary endpoint was to determine if a higher stem cell dose would result in a lower increase in symptom severity at 1 week post-ASCT. The area under the curve (AUC) of top 5 symptoms was calculated for each group using the trapezoidal rule.
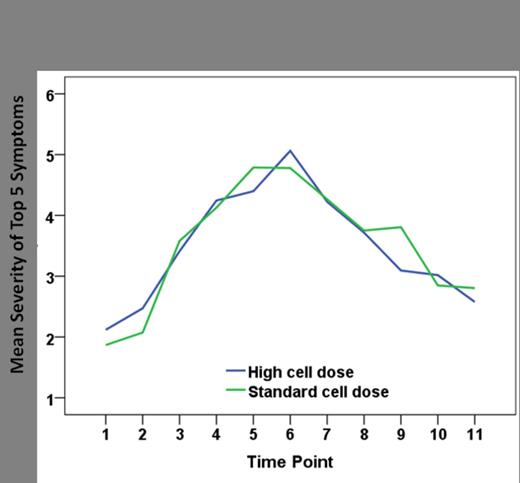
Between March 2008 and May 2013, 79 patients were enrolled, of which 73 patients were evaluable. 14 of these evaluable patients had light-chain amyloidosis (AL). 35 patients (48%) were randomized to receive a standard stem cell dose while 38 (52%) were randomized to the high stem cell dose arm. Patients were well-matched for age, gender, race, and ISS stage between the two groups. Disease responses prior to ASCT were 28.5% PR, 48.5 % VGPR + CR in the standard dose arm and 47.3 % PR, 31.5 % VGPR + CR in the high dose arm. The median CD34+ cell dose per kg was 4.915 e6 in the standard dose arm and 10.12 e6 in the high dose arm. Median time to ANC >500 was 10 days in both the standard dose arm and in the high dose arm. Similarly, median time to platelet engraftment (platelets >20,000) was 10 days in both arms. From pre-ASCT to day 7, the 5 symptoms with the largest increase were lack of appetite, drowsiness, fatigue, muscle weakness and disturbance in sleep. This severity did not differ between the 2 treatment arms (p=0.827). Over the 28 day course of ASCT the 5 symptoms with the greatest cumulative severity were fatigue, lack of appetite, drowsiness, disturbance in sleep and pain. Again, these increases were not different between the 2 treatment arms (p=0.882, Figure 1). AUCs of those top 5 symptoms did not show any significant difference between groups (228.4±191.0 for high cell dose group, 223.0±94.0 for standard cell dose, p=0.826).
Mean symptom severity throughout ASCT course. Time points 1-11 represent the period pre-SCT to day 28 after stem cell infusion.
Mean symptom severity throughout ASCT course. Time points 1-11 represent the period pre-SCT to day 28 after stem cell infusion.
Infusion of higher autologous stem cell dose after high dose chemotherapy does not yield a difference in engraftment time or symptom burden in the first few weeks after ASCT. Further correlative studies are in process to determine if there are any cytokine differences between the two cell dose arms.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal