Abstract
A rapid enumeration of CD34+ cells is expected to facilitate a safe and efficient PBSC collection for a successful transplantation. We previously reported a novel enumeration method of hematopoietic progenitor cells (designated as HPC) using an automated hematology analyzer, XN-series model (Sysmex Corporation, Japan), which does not require monoclonal antibody. This method seemed very promising in a pilot study in a single institute (ASH meeting #1922, 2011). Hence, we conducted a multicenter study to confirm the results in a larger number of subjects in various situations. In this study, we used an up-graded analyzer which had been installed with a revised analytical program. This new program employs an automatically-adjusted gating to fit the individual variety, whereas the former used a fixed gating for detecting HPC. We planned to divide the study into 2 parts; the first is to confirm the outcome of the previous single-institute study and to develop a model to predict CD34+ cell counts from HPC, and the second is to validate the model. Here we show the results of the first part of the study.
PB or PBSC samples were taken from G-CSF- and/or chemo-mobilized subjects (25 patients undergoing PBSCT and 25 healthy donors) in 5 institutes. Samples were divided into 2 tubes; one is for in-house assay in which HPC and CD34+ cells were counted in each institute within a few hours without any manipulation, and another was shipped to the central laboratory (SRL, Inc., Japan) where CD34+ cell count was examined. HPC and central labo-assayed CD34+ cell counts were compared with each other and used in a correlation analysis. HPC and in-house CD34+ cells were used in the analysis of kinetics. This study was approved by IRB, and an informed consent was obtained from each participant before apheresis.
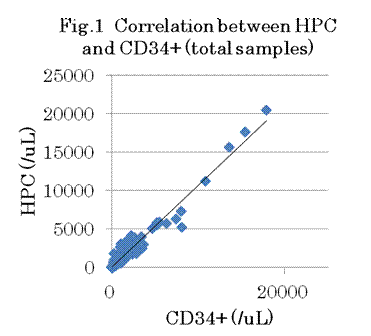
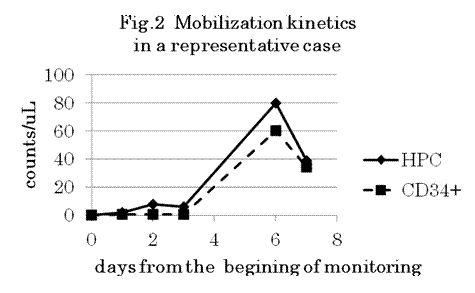
Five PBSC samples (2.3%) were estimated to be invalid for HPC assay because of difficulty in identifying HPC, and were excluded from the analysis. There were good correlations between HPC and CD34+ cell counts in all samples (n=211; R2 0.9576, slope 1.0678, Fig.1), in PB (n=87; R2 0.7631, slope 0.8878) and PBSC (n=124; R2 0.9501, slope 1.0689), in autologous PBSCT patients (n=116; R2 0.9759, slope 1.0596) and donors (n=95; R2 0.7914, slope 1.1381). However, HPC and CD34+ cell counts differ more than 3 times around the critical concentrations for making decision in some samples. Concerning their kinetics in PB during mobilization, which was observed in 2 donors and 9 patients, HPC showed a very similar trend as CD34+ cells, and the timing of appearance and increase in PB was almost concordant in most cases (Fig. 2).
In this multicenter study, we confirmed not only there was a good correlation between HPC and CD34+ cell counts, but also both HPC and CD34+ cell counts in PB showed a very similar trend during mobilization, as was observed in our previous single-institute study. However, a few samples were estimated as invalid for HPC assay, and there were a few more samples in which HPC and CD34+ cell counts differ significantly. Therefore, it remains to be elucidated how much HPC could be helpful in the clinical setting for determining the optimal timing of collection, for predicting the number of CD34+ cells in the apheresis product, and for determining the optimal blood volume to be apheresed. We are currently underway of the later validation part of this study to evaluate these issues.
Tanosaki:Sysmex Corporation: Automated hematology instruments were provided on loan and the cost for CD34+ cell counts were owed to Sysmex Corporation, Kobe, Japan in this study. Other.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal