Abstract
Historically lymphocyte (Lc) recovery following hematopoietic transplantation, immunosuppressive therapies, and infectious diseases has relied quite heavily on peripheral blood (PB) analysis. The PB, however, comprises less than 2% of total Lcs in the body, hence small changes in trafficking rates (e.g., lymphoid tissue (LT) homing) could have profound effects on PB Lc count. In this study we explored the use of a radiotracer rhesus IgG1 anti-CD4R1 Tc99mFab'2 and single photon emission computed tomography (SPECT/CT) to sequentially image CD4+ Lc recovery in the whole-body following total body irradiation (TBI) and autologous CD34+ cell transplant.
Three specific pathogen free rhesus macaques were used in this study. Each received 4Gy TBI on two sequential days (8Gy total). CD34+ cells were immunoselected from a leukapheresis product following cytokine mobilization over 5 days using G-CSF and Stem Cell Factor. 4-9x106/kg CD34+ cells were reinfused on the last day of irradiation after being transduced in culture once with a SIV/HIV chimeric lentiviral vector expressing EGFP at a multiplicity of infection of 50. SPECT/CT images were taken prior to transplant as a baseline; following mobilization with AMD3100 at least two months prior to transplant; and at approximately 6, 30, 90, 150 and 260 days post-TBI/transplant. Development of immunogenicity to the radiotracer was monitored using size exclusion high performance liquid chromatography (HPLC) and an in vitro cell binding assay.
Dynamic SPECT acquisition for two hours following mobilization with 1mg/kg AMD3100 SQ did not reveal measurable changes in the whole-body CD4 pool, despite an ∼ 2-fold maximum increase in CD4+ PB cell counts. This is consistent with a small percentage of CD4+ cells being released from tissues to PB without significantly affecting total Lc counts in the whole-body.
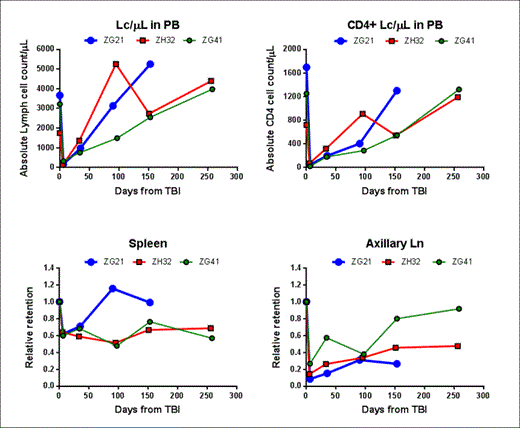
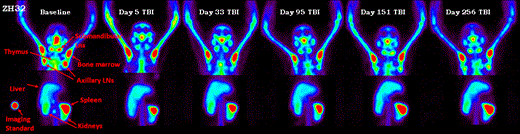
Following 4Gyx2 TBI, all 3 animals experienced dramatic depletion of PB CD4 counts (a 90-98% decrease from baseline) (Table 1). Variability in tissue CD4 depletion was observed between and within hosts, with more dramatic decreases observed in axillary lymph nodes (73-92%) and only mild decreases in the spleen (36-40%). Similarly, recovery of the CD4 pools in LTs following transplant varied between and within hosts and appeared overall slower than the dynamics of repopulation in the PB, suggesting changes in trafficking rates between LTs and PB has a role in transplant recovery. By month 9 post-TBI, one animal (ZG41) had full recovery of the PB CD4+ Lc count and the CD4 pool in axillary LNs quantified by the SPECT/CT, but minimal recovery of CD4 pools were observed in the spleen. In another animal (ZG21), full recovery of the PB CD4+ cell count was observed by month 5 concomitant with full recovery of the spleen CD4 pool, despite still greater than 70% depletion of CD4 pool in axillary LNs. In the last animal (ZH32), by month 3 the CD4+ Lc count in PB was fully recovered, however, minimal CD4 pool recoveries were observed in LT through month 9 post-TBI (Figure 1). EGFP expressing Lcs first appeared in the circulation on average(SD) 69(48) days following transplant and were CD8+ and CD20+. In all animals, CD4+ EGFP+ Lcs were not observed until after month 5, suggesting that endogenous recovery preceded graft recovery in the PB.
Of importance is that no immunogenicity to the radiotracer was detected despite sequential imaging up to month 5 in one animal and up to month 9 in the other two animals. This is in contrast to animals that were imaged several years post-transplant. In these animals immune complex formation to the radiotracer was observed by HPLC after a single exposure to the rhesus IgG1 anti-CD4R1 Tc99mFab'2. This suggests either tolerance was induced in animals exposed to the radiotracer shortly following transplant or that immune recovery remains incomplete after 150 days in these animals.
These results demonstrate for the first time that immune reconstitution of lymphoid tissues can be effectively monitored following transplant of autologous CD34+ cells and TBI, that the reconstitution appears to be discordant between PB and LT, as well as identifying a potential approach to decreasing immunogenicity to complex antigens through lymphocyte mobilization during antigen exposure.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal