Abstract
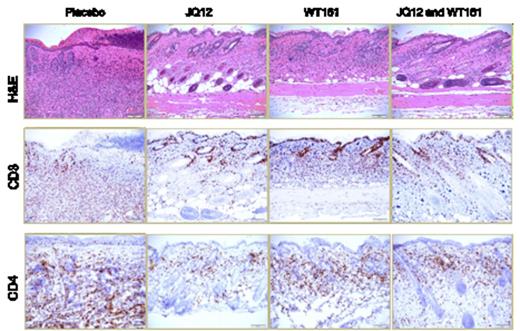
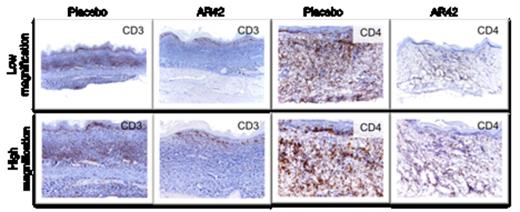
Cutaneous lymphoma is a heterogeneous group of neoplasms of skin-homing malignant lymphocytes. Cutaneous T-cell Lymphoma (CTCL) represents 70-80% of all cutaneous lymphoma and its pathogenesis is largely unknown. Previous studies have shown that interleukin (IL)-15 is a potent stimulant and growth factor for CTCL cells in vitro. In order to investigate the intrinsic levels of IL-15 in CTCL patients, malignant CD4+ T-cells were analyzed for expression of IL-15. Relative quantitation of IL-15 transcript in patient vs normal donor (ND) CD4+ cells showed overexpression of IL-15 in patients (fold increase mean ± SD = 5.36 ± 4.39, N=13, P<0.001). Increase in IL-15 transcript was directly proportional to disease severity in patients i.e. fold increase mean ± SD in IL-15 in Stage I =3.28 ± 1.42, N=3 each, P=0.0047 vs. Stage III patients = 7.42 ± 1.30, N=3 each, P=0.0073. Further, cutaneous lesions in patients stained positive for IL-15 protein in atypical lymphoid cells and Pautrier's microabscess. We next investigated the role of IL-15 in CTCL development using IL-15 transgenic (tg) mice. Within 4-6 weeks of birth, IL-15 tg mice developed extensive patch/plaque skin lesions, progressive alopecia, and severe pruritus. Adult IL-15 tg mice developed extensive involvement with cutaneous lymphoma that was fatal in 100% of the mice (P=0.0003). Antibodies staining revealed that CD4+ skin resident T-cells in IL-15 tg mice were CD3+CD62L-CD44hiCCR4+CLA+. Flow cytometric analysis of single cell suspension of skin showed ∼25-fold increase in CD3+ T-cells in IL-15 tg compared to WT controls (Mean ± SD of absolute number of cells= 3.80 ± 6.97, N=14 vs. 0.15 ± 0.26, N=8 respectively, P<0.001). Lymphoma cells from IL-15 tg mouse skin engrafted and mimicked the primary disease in immune deficient SCID mice upon adoptive transfer. CD4+ T-cells from CTCL patients showed increased histone deacetylases (HDAC) 1, HDAC2 and HDAC6 transcripts over ND CD4+ T-cells and immunoblot analysis of ND CD4+ T-cells exposed to 100ng/ml IL-15 showed upregulation of HDAC1, HDAC2 and HDAC6 ex vivo. IL-15 stimulation of ND CD4+ T-cells resulted in loss of expression of the downstream HDAC1/2 target tumor suppressor, p21 in vitro, and knock down of HDAC6 in IL-15 stimulated ND CD4+ T-cells inhibited their migration in vitro; suggesting that IL-15 mediated upregulation of HDAC6 is critical for T-cell migration. Considering these observations, we used specific novel HDAC inhibitors (HDACi) to target HDAC1/2 (JQ12) and/or HDAC6 (WT161) in IL-15 tg mice to determine if we could prevent CTCL in vivo. IL-15 tg mice were treated with 50mg/kg of either or both the inhibitors, 5 days/week for 4 weeks (n=4 each). While placebo treated IL-15 tg mice progressively developed lesions during the course of treatment, IL-15 tg mice treated with JQ12 and/or WT161 showed no clinical signs of disease. This was further corroborated by histopathology analysis of skin sections from treated mice (Figure 1). Thus, our data suggest that inhibiting HDAC1, HDAC2 and/or HDAC6 pathways inhibits the development of CTCL in IL-15tg mice. In addition to the prevention study, we assessed the ability of a novel pan-HDACi, AR42, to treat active and progressive disease in our model. IL-15 tg mice with established CTCL were randomized to receive either AR42 or placebo feed (n=6 each) for 12 days. The IL-15 tg mice treated with AR42 showed remarkable improvement compared to the placebo mice whose disease progressed. Histopathology analysis of the AR42-treated IL-15 tg mice showed an impressive clearance of the CD3+ and CD4+ atypical lymphocytic infiltrate compared to placebo-treated mice (Figure 2). In summary we provide evidence that IL-15 has a causal role in the pathogenesis of CTCL; that IL-15 tg mice provide a novel model for studying disease pathogenesis and for evaluating potential therapies; that HDACi targeting specific HDACs may be effective in preventing CTCL and a novel pan-HDACi can reverse severe dermatologic disease in this CTCL model.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal