Abstract
B-UCL is a new category of lymphomas that comprises a heterogeneous group of tumors from a morphological, phenotypic and genetic perspective with features intermediate between DLBCL and BL. This entity has been included as a new category in the 2008 World Health Organization (WHO) Classification of Tumors of Hematopoietic and Lymphoid Tissues. The outcome of patients with B-UCL is generally considered very poor. However, the number of series analyzing the prognosis of B-UCL is scarce and they include small number of patients not homogeneously treated. Against this background, herein we present a series of patients diagnosed with B-UCL (WHO 2008) or Burkitt-like variant (WHO 2001) homogeneously treated according to the PETHEMA-Burkimab-04 trial. This trial was designed for the treatment of BL, including both HIV positive and negative patients. Patients with B-UCL were allowed to be treated following the same protocol and were considered separately. The main clinical characteristics and outcome were compared to those obtained from classical BL patients in the same trial (Ribera et al. Cancer 2013).
Diagnosis of B-UCL was made in each center based on morphological, phenotypic and genetic characteristics and centrally reviewed by two pathologists. Patients received 6 courses of chemotherapy including continuous methotrexate infusion, cytarabine, vincristine and dexametasone among other drugs. A single dose of rituximab (375mg/m2) before each cycle was administered. Patients with non-bulky stage I-II disease received only 4 courses of treatment, whereas patients with advanced stage (II bulky-IV) received 2 additional doses of rituximab at the end of the chemotherapy.
A total of 30 patients with B-UCL were included and were compared with the 118 patients with BL. Median follow-up for survivors was 24 months (range 3-93). Considering B-UCL, 8 (27%) patients were HIV positive and 10 (33%) had an ECOG performance status ≥ 2. Four (13%) and 6 (20%) patients had CNS involvement and bulky (>10 cm) disease, respectively. Serum LDH level was > UNL in 25 (83%) patients. These and other clinical characteristics were similar between patients with B-UCL and BL, except for the median age at diagnosis (57 years in B-UCL vs. 44 years in BL, p=0.001). In B-UCL patients, death during induction occurred in 5 patients (17%) and 2 patients experienced treatment failure. Twenty-three (77%) patients achieved complete remission. Relapse after CR and death in remission were observed in one patient, each. All these outcomes were comparable with BL patients except for a trend to a higher risk of death during induction in patients with B-UCL (17% in B-UCL, vs. 8% in BL, p= 0.1). A total of 104 consolidation cycles were evaluable for patients with B-UCL. Most frequent grade 3-4 toxicities were neutropenia (n=57, 54%), thrombocytopenia (n=44, 42%) and infection (n=21, 20%). Grade 3-4 gastrointestinal, renal and hepatic toxicities were seen in <5% of the cycles.
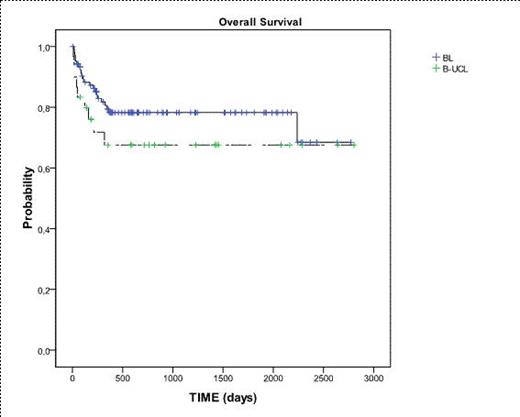
Overall survival from diagnosis in patients with B-UCL and BL treated with Burkimab-04.
Overall survival from diagnosis in patients with B-UCL and BL treated with Burkimab-04.
Baseline characteristics of all patients according to definitive diagnosis.
| Characteristics . | Burkitt (n = 118) . | LDCG-B/Burkitt (n=30) . | p . | |
|---|---|---|---|---|
| Gender, male n (%) | 85 (72) | 21 (70) | 0.8 | |
| Age, median [min -max] | 44 [15 - 83] | 57 [16-77] | 0.001 | |
| Stage, n (%) | Non bulky I – II | 26 (22) | 10 (33) | 0.8 |
| II (bulky), III - IV | 92 (78) | 20 (67) | ||
| ECOG ≥2, n (%) | 55 (47) | 10 (33) | 0.3 | |
| HIV infection | 39 (33) | 8 (27) | 0.4 | |
| Extranodal involvement (≥2 sites), n (%) | 55 (47) | 11 (37) | 0.5 | |
| CNS involvement, n (%) | 14 (12) | 4 (13) | 0.6 | |
| Bulky disease, n (%) | 31 (26) | 6 (20) | 0.3 | |
| LDH level above normal, n (%) | 106 (90) | 25 (83) | 0.5 | |
| Age-adjusted IPI, n (%) | Low | 6/116 (5) | 2 (7) | 1 |
| Low-intermediate | 17/116 (15) | 6 (20) | ||
| Intermediate-high | 46/116 (40) | 11 (37) | ||
| High | 47/116 (40) | 11 (37) | ||
| Follow-up [all patients] (months) | 19 (0-92) | 16 (0-93) | 0.9 | |
| Characteristics . | Burkitt (n = 118) . | LDCG-B/Burkitt (n=30) . | p . | |
|---|---|---|---|---|
| Gender, male n (%) | 85 (72) | 21 (70) | 0.8 | |
| Age, median [min -max] | 44 [15 - 83] | 57 [16-77] | 0.001 | |
| Stage, n (%) | Non bulky I – II | 26 (22) | 10 (33) | 0.8 |
| II (bulky), III - IV | 92 (78) | 20 (67) | ||
| ECOG ≥2, n (%) | 55 (47) | 10 (33) | 0.3 | |
| HIV infection | 39 (33) | 8 (27) | 0.4 | |
| Extranodal involvement (≥2 sites), n (%) | 55 (47) | 11 (37) | 0.5 | |
| CNS involvement, n (%) | 14 (12) | 4 (13) | 0.6 | |
| Bulky disease, n (%) | 31 (26) | 6 (20) | 0.3 | |
| LDH level above normal, n (%) | 106 (90) | 25 (83) | 0.5 | |
| Age-adjusted IPI, n (%) | Low | 6/116 (5) | 2 (7) | 1 |
| Low-intermediate | 17/116 (15) | 6 (20) | ||
| Intermediate-high | 46/116 (40) | 11 (37) | ||
| High | 47/116 (40) | 11 (37) | ||
| Follow-up [all patients] (months) | 19 (0-92) | 16 (0-93) | 0.9 | |
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal