Abstract
Variability in the severity of iron overload among homozygotes for the HFE C282Y polymorphism is one of the major problems extant in our understanding of hereditary hemochromatosis (HH). We conducted exome sequencing of DNA from C282Y homozygotes with markedly increased iron stores (cases) and C282Y homozygotes with normal or mildly increased iron stores (controls) to identify rare and common causal variants associated with variability of disease expression in HH. Criteria for cases included serum ferritin >1000 µg/L at diagnosis, and (a) mobilized body iron >10 g by quantitative phlebotomy, and/or (b) hepatic iron concentration >236 µmol/g dry weight. Criteria for controls included (a) serum ferritin <300 µg/L, or (b) age ≥50 y with ≤3.0 g iron removed by phlebotomy or age ≥40 y with ≤2.5 g iron removed by phlebotomy to achieve serum ferritin <50 µg/L. Deep sequencing of the full exome was performed in 33 cases and 14 controls. After quality control filtering, the dataset included 82,068 SNPs and 1,403 insertions/deletions (indels).
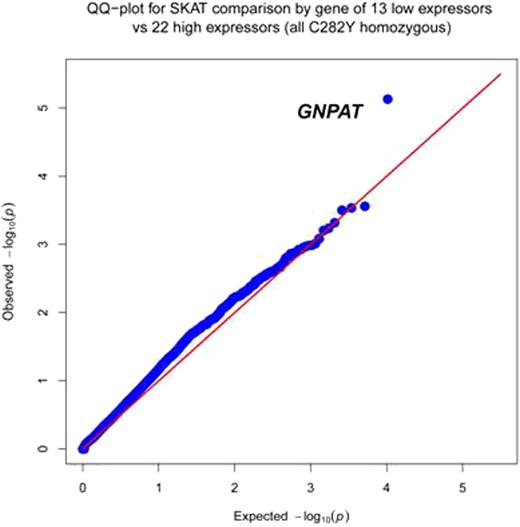
Our initial analysis tested for differences in the distribution of variants between groups for each gene separately using the Sequence Kernel Association Test (SKAT) that includes rare and common variants but downweights the contribution of common variants to the test statistic. Only non-synonymous variants were included in the by-gene tests. Principal components were constructed from the exome variants to adjust for possible confounding by ancestry and to confirm no ancestral outliers.
Inspection of the two variants contributing to the GNPAT by-gene p-value revealed one missense variant (rs11558492) for which 0/13 controls had a polymorphism, while 16/22 cases had at least one missense variant, and one case was homozygous for this missense variant. The latter case presented at the early age of 26 with a serum ferritin of 1762 µg/L, 4+ hepatocellular iron and hepatic iron concentration of 284.4 µmol/g dry weight. GNPAT (aka DHAPAT) mutations/deletions have been found in peroxisomal disease, a class of diseases in which increased hepatic iron is observed (Biochim Biophys Acta 1801:272-280, 2010). GNPAT rs11558492 is common among people of European descent but might interact with aberrant HFE to increase risk of hepatic iron overload. Three rare variants in CDHR2 accounted for its low p-value, having a cumulative frequency of 4/13 among controls and 0/22 among cases: rs115050587, rs752138, rs143224505 with minor allele frequencies, MAF = 1.4%, 4.7% and 0.06%, respectively. The first two polymorphisms are predicted to be highly damaging by PolyPhen2 and the third probably damaging. Expression levels of CDHR2 recently have been associated with increased hepatocyte iron and elevated serum ferritin in liver allograft patients (J Clin Invest 122:368-382, 2012).
These data indicate associations between iron status in HFE C282Y homozygotes and genes with previous links to iron overload that may modify severity of disease expression. Of note, the data suggest that more than one modifier gene may be involved in determining severity of disease in HFE C282Y homozygotes. Our results identify candidate genes for expanded studies that would examine their functional significance for iron absorption and metabolism.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal