Abstract
Diffuse large B-cell lymphoma (DLBCL) is the most common lymphoma with >40,000 cases annually in the US. While most patients are cured by frontline immunochemotherapy, up to 20% recur and salvage therapy is poor. Relapse detection relies on CT imaging, which is hampered by radiation exposure, high costs, high false positive rates, and infrequently detects relapse prior to symptoms. Novel methods to detect early relapse may improve outcomes. The LymphoSIGHT™ platform is a high-throughput DNA sequencing method that can detect minimal residual disease (MRD) of lymphoid malignancies in peripheral blood (PB) (Faham et al. Blood 2012) with a sensitivity of one lymphoma cell per million leukocytes. We showed it can also detect circulating tumor DNA in the PB of DLBCL pts (Armand Br J Haematol 2013). We assessed the sensitivity and specificity of LymphoSIGHT™ to detect circulating tumor DNA in serum after frontline therapy in DLBCL.
All pts were newly diagnosed DLBCL undergoing frontline EPOCH-based chemotherapy. Post-therapy surveillance included clinic visits, PB samples, and contrast CT scans at 3, 4, 6, 12 and 12 mos intervals for the first 5 years, respectively. Pre-treatment lymph node biopsies and PB samples collected at initial diagnosis and during surveillance visits were sent for VDJ amplification and MRD sequencing assessment. Using universal primer sets, we amplified immunoglobulin heavy and kappa chain (IGH and IGK) variable, diversity, and joining gene segments from genomic DNA in tumor biopsy and PB samples. Amplified products were sequenced, and tumor-specific clonotypes were identified for each pt based on their high frequency within the B-cell repertoire in the lymph node. The presence of the tumor-specific clonotype was then quantitated in PB samples.
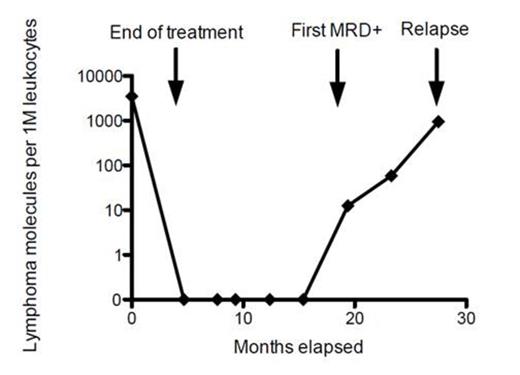
Twenty-seven pts, treated between July 1996 and July 2005, had adequate DNA from pre-treatment FFPE lymph node biopsies, and 25 (92.6%) had a high-frequency clonal rearrangement (MRD) detected. The median pt age was 49 years (20-75) and an IPI risk groups included 8 low, 3 low-intermediate, 11 high-intermediate and 3 high. The pts had a median of 12 surveillance CT scans (range 1-13) and 221 serum samples analyzed for MRD. Two pts progressed early after cycles 1 and 6. The first pt had progressive disease by CT scan after cycle 1 and remained MRD positive. The second pt remained MRD positive at the end of cycle 6, at which time a CT showed CR-unconfirmed, and progressed one month later. In the remaining 23 pts who achieved CR, seven relapsed at a median of 20.3 (range 5.6-151.6) months. The 16 pts in sustained CR had a median follow-up of 99.7 months (range 3.0-136.3) and none were MRD positive post-treatment (specificity 100%; 88.9-100%). There were 0 false positives in 200 post-treatment samples tested (specificity 100%, 99-100%). Of 7 relapsing pts, 6 had serum samples within 1 year of recurrence. Of these, 5 pts had a positive MRD (range 3.2 to 11.2 months) (sensitivity 83%; 44-98%) at a median of 9.2 months (range 3.2 to 11.2 months) prior to a positive CT scan (Figure). One relapsing pt only had a serum sample obtained 18 mos before CT recurrence and it was MRD negative.
Our data suggest that a DNA sequencing-based MRD test of serum can predict clinical relapse with high sensitivity and specificity in DLBCL prior to CT progression. MRD assay detection may significantly reduce the cost, inconvenience and toxicity of surveillance. Importantly, early detection of recurrence may significantly improve pt outcomes. Additional pts are being analyzed and updated results will be available.
This work was supported by the Intramural Research Program of NCI at NIH and Sequenta, Inc.
Kong:Sequenta: Employment, Equity Ownership. Zheng:Sequenta: Employment, Equity Ownership. Willis:Sequenta: Employment, Equity Ownership, Membership on an entity’s Board of Directors or advisory committees. Faham:Sequenta: Employment, Equity Ownership, Membership on an entity’s Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal