Abstract
Our previous studies have shown little or no difference in the overall survival (OS) of urban over rural cohorts with lymphoma. Autologous hematopoietic stem cell transplantation (Auto-HSCT) is a frequently used treatment for non-Hodgkin lymphoma (NHL), but the elderly tend to tolerate transplant less well than their young counterparts. Increased prevalence of chronic conditions, comorbidity and frailty, serve to further complicate treatment in the elderly. We hypothesize that the same factors that contribute to the complexity of Auto-HSCT in the elderly may also contribute to outcome disparity according to area of residence.
To determine if area of residence is an independent risk factor for the following clinical outcomes: relapse, non-relapse mortality (NRM), disease-free survival (DFS) and OS following Auto-HSCT in the elderly with NHL.
This is a retrospective cohort study of patients (pts) age ≥60y who underwent first Auto-HSCT for NHL between 1985 and 2012. Using pts' residential ZIP codes at the time of transplant, the primary area of residence was categorized as urban or rural according to the Rural-Urban Commuting Area Codes classification system. Multivariate analyses (MVA) were performed using Cox proportional hazards regression analysis to evaluate the association between area of residence and all outcomes while adjusting for significant patient-, disease-, and treatment-related variables.
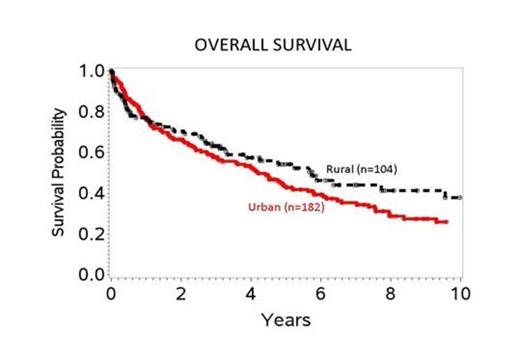
During the study period, 1616 pts underwent Auto-HSCT for NHL; 321 (20%) of whom were elderly. A total of 286 (89%) had classifiable U.S. ZIP codes: urban (n=182, 64%) and rural (n=104, 36%). The median age was 65y (range 60-77), 64% males, and 93% of the NHL types are high-grade. We failed to detect significant differences in all patient-, disease-, and treatment-related factors between the two cohorts except for disease stage at the time of transplant (p=0.03); urban pts tend to be in relapse at the time of transplant (35% vs 25%), while rural pts tend to be in second or more complete remission ( 34% vs 18%). MVA results are summarized in the table below. We failed to detect differences in the risk of relapse and NRM between urban and rural cohorts. Both risk for treatment-failure (inverse of DFS) and mortality were time-dependent such that the association between area of residence and outcome varied before and after 6 months post-transplant. The risk of treatment-failure and mortality was significantly lower in the rural cohort compared to the urban cohort after 6 months; while similar in the first 6 months. Age and year of transplant were significantly associated with DFS and OS; disease stage at the time of transplant was not significant. The probability of OS (see figure) at 5 and 10 years post-transplant in urban and rural cohorts were 44% vs 54% and 26% vs 38%, respectively. The causes of death were not statistically different between the two groups with most dying from relapse, multi-organ failure and second malignancies.
The absence of clear differences in characteristics of elderly NHL pts who underwent Auto-HSCT according to area of residence suggests a similar selection process. The better DFS and OS after 6 months that persist over time and is not explainable by marked differences in relapse, NRM or treatment received suggests interplay of other non-biological factors including: attitudes, health seeking behaviors, environment-related factors, or health care follow-up. Further research is needed to determine the underlying cause of the observed outcome disparity among elderly NHL transplant pts.
Armitage:Ziopharm: Consultancy; Roche: Consultancy; Genetech : Consultancy; Seattle Genetics: Consultancy; GlaxoSmith Kline : Consultancy; Tesaro bio, Inc. : Membership on an entity’s Board of Directors or advisory committees. Vose:Sanofi-Aventis US, Inc.: Research Funding; Pharmacyclics: Research Funding; Onyx Pharmaceuticals: Research Funding; Millennium: Research Funding; Janssen Biotech : Research Funding; Incyte Corp.: Research Funding; GlaxoSmithKLINE: Research Funding; Genentech: Research Funding; Celgene: Research Funding; Bristol-Myers Squibb: Research Funding; Allos Therapeutics/Spectrum: Research Funding; US Biotest, Inc. : Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal