Abstract
Phase 2 Trial of GS-9973, a selective Syk inhibitor, has Activity in Subjects with Chronic Lymphocytic Leukemia (CLL)
Spleen tyrosine kinase (Syk) is an essential mediator of B-cell receptor signaling in normal and transformed B-cells. GS-9973 is an orally bioavailable, small-molecule, selective inhibitor of Syk. The Kd of GS-9973 for Syk was 7.6 nM with no other kinase < 100 nM (Ambit scanMax at 10 mM).
This Phase 2 trial enrolled subjects with CLL (1 cohort) or NHL (4 cohorts) of 40 subjects each. All subjects were treated with GS-9973 800 mg BID. Tumor imaging was conducted at weeks 8, 16, 24 and every 12 weeks thereafter. Response was evaluated according to standard criteria for CLL (Hallek 2008 and Cheson 2012). GS-9973 plasma levels were obtained throughout the study and concurrently obtained circulating CD5+CD19+ leukemic cells were assessed for changes in pSyk, pBLNK, pBTK and pAKT expression using a PhosFlow protocol. Chemokine/cytokine plasma levels were assessed using multiplexed bead suspension arrays.
This study initiated in March 2013. At time of data lock, 56 subjects with CLL/SLL (32) or NHL(24) have been enrolled. 18 subjects with CLL/SLL and 16 subjects with NHL (10 iNHL, 3 DLBCL and 3 MCL) have completed ≥ 4 weeks of treatment and are included in the safety analysis. Median age was 71 (range 55 - 88), 65% were male. The median number of prior treatment regimens was 5 (range 1-14). All CLL/SLL subjects had received an anti-CD20 antibody, 89% had received an alkylating agent (56% had bendamustine) and 72% had received fludarabine.
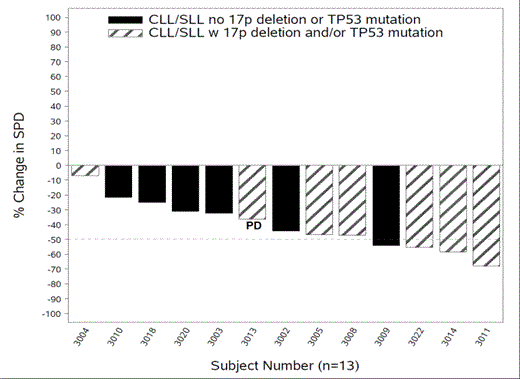
Investigator assessed week 8 efficacy analysis is available for 22/54 patients which included 13 CLL/SLL subjects, 7 of whom had 17p deletions and/or TP53 mutations. At the week 8 evaluation all subjects experienced reduced tumor bulk: 4 subjects achieved a decrease of > 50% in their measurable lymph node disease; 8 had < 50% decrease. One subject with a marked decrease in peripheral lymphadenopathy had unequivocal progression of non-measurable mediastinal disease and was considered to have disease progression. The waterfall plot is provided; the striped bars represent those CLL/SLL subjects with a 17p deletion and/or TP53 mutation.
GS-9973 was generally well tolerated. 30 of 34 (88%) of subjects experienced an adverse event. All treatment emergent adverse events occurring in ≥ 10% of 34 subjects are listed in the table. Reversible Grade 3 or 4 transaminase elevations occurred in 4 subjects. Two of the 34 subjects who received at least 1 dose of GS-9973 died while on study: 1 from progressive disease, 1 from pneumonia that occurred after 7 days on study.
| . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . | All Grades . |
|---|---|---|---|---|---|
| Fatigue | 7 | 5 | 2 | 14 (41%) | |
| ALT elevation | 5 | 1 | 3 | 1 | 11 (32%) |
| Nausea | 8 | 2 | 1 | 11 (32%) | |
| Diarrhea | 8 | 8 (24%) | |||
| Headache | 5 | 2 | 7 (21%) | ||
| Cough | 3 | 3 | 6 (18%) | ||
| Constipation | 5 | 1 | 6 (18%) | ||
| Insomnia | 4 | 1 | 5 (15%) | ||
| Pyrexia | 4 | 1 | 5 (15%) | ||
| Chills | 5 | 5 (15%) | |||
| Dizziness | 5 | 5 (15%) | |||
| Dehydration | 1 | 3 | 4 (12%) | ||
| Decreased appetite | 2 | 2 | 4 (12%) | ||
| Dyspepsia | 2 | 2 | 4 (12%) |
| . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . | All Grades . |
|---|---|---|---|---|---|
| Fatigue | 7 | 5 | 2 | 14 (41%) | |
| ALT elevation | 5 | 1 | 3 | 1 | 11 (32%) |
| Nausea | 8 | 2 | 1 | 11 (32%) | |
| Diarrhea | 8 | 8 (24%) | |||
| Headache | 5 | 2 | 7 (21%) | ||
| Cough | 3 | 3 | 6 (18%) | ||
| Constipation | 5 | 1 | 6 (18%) | ||
| Insomnia | 4 | 1 | 5 (15%) | ||
| Pyrexia | 4 | 1 | 5 (15%) | ||
| Chills | 5 | 5 (15%) | |||
| Dizziness | 5 | 5 (15%) | |||
| Dehydration | 1 | 3 | 4 (12%) | ||
| Decreased appetite | 2 | 2 | 4 (12%) | ||
| Dyspepsia | 2 | 2 | 4 (12%) |
The absolute lymphocyte count in CLL/SLL subjects increased a mean of 200%, by day 8 and then declined. Decreases in pSyk levels occurred in leukemic cells from 8 of 11 CLL/SLL subjects. The results of the disease-associated chemokines/cytokines assays are pending.
GS-9973 given on this dose and schedule was generally well tolerated. At the initial 8-week response assessment GS-9973 demonstrated activity in subjects with CLL/SLL, including those with poor prognostic features. Updated data on the larger cohort of CLL/SLL subjects will be presented.
Sharman:Gilead Sciences: Research Funding. Hawkins:Gilead Sciences: Employment, Equity Ownership. Hu:Gilead Sciences: Employment, Equity Ownership. Reddy:Gilead Sciences: Employment, Equity Ownership. Jin:Gilead Sciences: Employment, Equity Ownership. Melchor-Khan:Gilead Sciences, Inc.: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal