Abstract
Thrombocytopenia in myelodysplastic syndrome (MDS) occurs in approximately 50% of patients with low/intermediate-1 MDS and is associated with reduced survival; few therapies are available. In a randomized placebo-controlled study, 250 patients with MDS were randomized 2:1 to receive weekly romiplostim or placebo. In the original June 2011 analysis, romiplostim reduced clinically significant bleeding events (HR 0.83, 95% CI: 0.66, 1.05, P = 0.13) and platelet transfusions (RR 0.77, 95% CI: 0.66, 0.88), and increased IWG HI-P incidence (OR 15.6, 95% CI: 4.7, 51.8). Increases in peripheral blast counts to >10% were more frequent with romiplostim (25/167, 15%) than placebo (3/83, 3.6%), and in most cases resolved after discontinuation. Due to concerns of the data monitoring committee that the potential benefit seen in the reduction of bleeding did not outweigh the potential risk for disease progression to acute myeloid leukemia (AML) and that transient increases in blast cell counts might put patients at risk for diagnosis of and treatment for AML, treatment with study drug was discontinued in February 2011. Patients were then moved into the long-term follow-up (LTFU) portion of the study. Previously reported (July 2012) 58-week incidence of AML was romiplostim: 6.0% (10 patients), placebo: 4.9% (4 patients), HR 1.20 (95% CI: 0.38, 3.84). This report provides data on long-term follow-up of these patients to March 2013, with a particular emphasis on AML incidence.
Eligible patients had IPSS low/intermediate-1 MDS and platelets 1) ≤20x109/L or 2) ≤50x109/L with a history of bleeding, and were receiving only supportive care. Disease progression to AML was defined as 1) ≥20% blasts in the bone marrow or peripheral blood after 4 weeks following discontinuation of romiplostim, 2) pathology consistent with leukemia including chloroma or leukemia cutis, or 3) anti-leukemic treatment initiation. Results are presented by treatment group.
Of 250 patients in the study, 210 entered LTFU, and 98 of these patients remained on study as of March 2013; the median (Q1, Q3) follow-up was 25.5 (10.8, 33.1) months. Reasons for discontinuation during LTFU were similar in the romiplostim and placebo groups: death (46.7%, 98 patients); lost to follow up (4.8%, 10 patients), and consent withdrawal (1.9%, 4 patients).
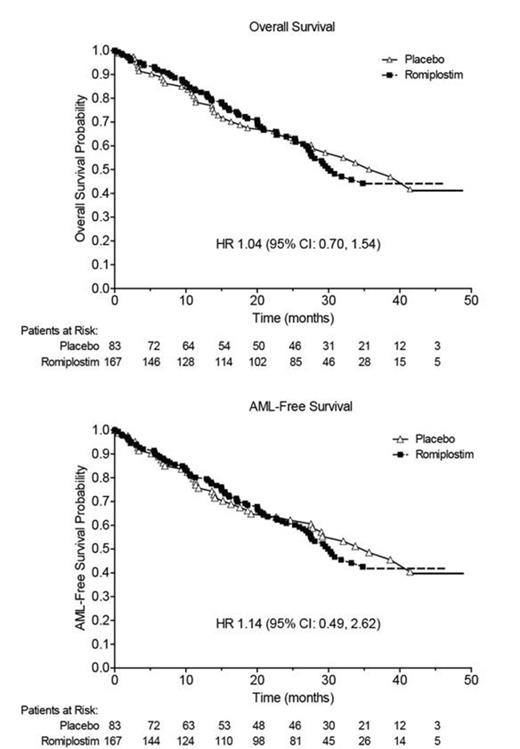
During the active study period or during LTFU, death was reported in 44.3% (74 patients) in the romiplostim group and 44.6% (37 patients) in the placebo group (HR 1.04, 95% CI: 0.70, 1.54) (Figure). AML was reported in 10.7% (18 patients) in the romiplostim group and 9.8% (8 patients) in the placebo group (HR 1.14, 95% CI: 0.49, 2.62) (Figure). The proportions of patients who either died or developed AML were 46.1% (77 patients) in the romiplostim group and 45.8% (38 patients) in the placebo group (HR 1.07, 95% CI: 0.72, 1.58). Thirteen of the 26 AML cases occurred in patients who were RAEB-1 and 5 cases were diagnosed by anti-AML treatment alone (Table). In LTFU, patient-reported use of MDS therapy (e.g., azacitidine or cyclosporine) in the romiplostim group was 40.6% and in the placebo group, 28.2%. Reported use of AML therapy (e.g., chemotherapy) was 8.8%, romiplostim and 7.0%, placebo.
Progression to AML to Date
| Study-defined AML . | Romiplostim N = 18 . | Placebo N = 8 . | Total N = 26 . |
|---|---|---|---|
| Baseline IPSS classification | |||
| RAEB-1/-2 | 10 (55.6%) | 3 (37.5%) | 13 (50.0%) |
| Non-RAEB | 8 (80.0%) | 5 (62.5%) | 13 (50.0%) |
| AML diagnosis by | |||
| Bone marrow/peripheral blast >20% | 15 (83.3%) | 6 (75.0%) | 21 (80.7%) |
| Anti-AML therapy alone | 3 (16.7%) | 2 (25.0%) | 5 (19.2%) |
| Study-defined AML . | Romiplostim N = 18 . | Placebo N = 8 . | Total N = 26 . |
|---|---|---|---|
| Baseline IPSS classification | |||
| RAEB-1/-2 | 10 (55.6%) | 3 (37.5%) | 13 (50.0%) |
| Non-RAEB | 8 (80.0%) | 5 (62.5%) | 13 (50.0%) |
| AML diagnosis by | |||
| Bone marrow/peripheral blast >20% | 15 (83.3%) | 6 (75.0%) | 21 (80.7%) |
| Anti-AML therapy alone | 3 (16.7%) | 2 (25.0%) | 5 (19.2%) |
Following the 2011 decision to stop study drug, results have been updated with more observational time on study. Specifically, the HRs for death or progression to AML are 1.04 and 1.14, respectively, compared with 2012 HRs of 1.09 (95% CI: 0.71, 1.68) and 1.15 (95% CI: 0.47, 2.85) respectively. In LTFU, more patients in the romiplostim group than in the placebo group received MDS therapy. As LTFU continues, additional data will be evaluated. Safety concerns regarding risk of disease progression to AML are still being investigated.
Kantarjian:Amgen Inc.: Research Funding. Off Label Use: Romiplostim is indicated for treatment of thrombocytopenia in patients with chronic ITP who have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy. Treatment of thrombocytopenia due to MDS, which is the subject of a study described in this abstract, is not an approved indication. Mufti:Novartis: Speakers Bureau; Celgene: Research Funding, Speakers Bureau; GlaxoSmithKline: Consultancy, Speakers Bureau; Amgen: Speakers Bureau. Fenaux:Amgen Inc.: Research Funding. Sekeres:Amgen Inc.: Membership on an entity’s Board of Directors or advisory committees; Celgene: Membership on an entity’s Board of Directors or advisory committees. Szer:Alexion Australia: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees; Celgene: Membership on an entity’s Board of Directors or advisory committees; Sandoz: Membership on an entity’s Board of Directors or advisory committees; Pfizer: Membership on an entity’s Board of Directors or advisory committees. Platzbecker:Amgen Inc.: Honoraria. Kuendgen:Celgene: Research Funding. Gaidano:Amgen Inc.: Consultancy, Honoraria. Wiktor-Jedrzejczak:Amgen Inc.: Research Funding. Meibohm:Amgen Inc.: Consultancy. Lopez:Amgen Inc.: Employment, Equity Ownership. Giagounidis:Amgen Inc.: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal