Differentiation therapy with all-trans retinoic acid (atRA) has markedly improved the outcome of acute promyelocytic leukemia (APL) but has had little clinical impact in other AML sub-types. Cell intrinsic mechanisms of atRA resistance have been previously reported (i.e., over-expression of PRAME or up-regulation of Tal1 pathway), yet the majority of AML blasts are sensitive to atRA in vitro. Even in APL, although atRA induces complete remissions (CRs) in >80% of cases, most patients eventually develop disease recurrence. We have recently showed that BM stromal control of RA signaling via expression of CYP26 is important in determining normal hematopoietic stem cell fate. Thus, here we hypothesize that the BM microenvironment is responsible for the difference between the in vitro sensitivity and in vivo resistance of AML to atRA-induced differentiation. It is well-recognized that pharmacological concentrations of atRA (10-7M x 3 days) differentiates NB-4 cells [a t(15;17) APL line] as demonstrated by decreased cellular expansion (32.9±1 vs. 3.5±1.3, mean±SD of cellular expansion folds of control vs. atRA-treated cultures, n=3, p<0.01) induction of cell cycle arrest (52.7% vs. 86.2% Go/G1, control vs. atRA), decreased clonogenic activity (61±12% of control in atRA cultures, n=3, p<0.01), increased expression of differentiation markers (47.4±7% vs. 84.5±13% CD11b positive cells, control vs. atRA, n=3, p=0.01), disappearance of blasts (44.5±7% vs 24.3±4% blasts, control vs. atRA, n=4, p<0.01) and emergence of morphological neutrophils. We observed similar pro-differentiation effects of atRA on other AML lines including HL-60 [non-t(15:17) APL line], KG-1 (primitive line arising from MDS), Kasumi-1 [t(8;21)] and OCI-AML3 (NPM1 mutated). When the incubations were repeated with the 5 AML lines in the presence of BM stroma, atRA activity (i.e., upregulation of differentiation markers, inhibition of clonogenic growth) was blocked. Inhibition of stromal CYP26 by the competitive inhibitor R115866, rescued the AML cell sensitivity to atRA: clonogenic recovery of NB4 cells was 92±17% on stroma with atRA compared to 56±15.9% when R115866 was added) (mean±SD of clonogenic activity of untreated stroma cultures, n=3, p=0.03) and 67.5±8.9% cells expressed the differentiation marker CD11b when treated with atRA on stroma compared to 95.1±4.9% with the combination of atRA+R115866 (n=3, p=0.03). Similar results were observed using HL-60, KG-1, Kasumi-1 and OCI-AML3 cells. CD34+ AML blasts from the BM of newly-diagnosed patients with t(8;21) AML were cultured as above. When isolated from the BM, the blasts showed only low-level expression of mature myelomonocytic markers (n=3). However, the expression of differentiation markers was significantly increased when cultured in serum (which contains about 10-9M atRA) and even further with the addition of 10-7M atRA (Figure). The presence of stroma during culture was associated with maintenance of an immature leukemic phenotype; moreover, as seen above, inhibition of CYP26 by R115866 restored AML sensitivity to atRA (Figure). CYP26 inhibitor had no effect on AML cells in stroma independent cultures. Our data suggest that CYP26 activity in the BM microenvironment creates retinoid low sanctuaries that protect AML cells from systemic atRA therapy. Thus, inhibition of CYP26 activity provides a new opportunity to expand the clinical activity of atRA in both APL and non-APL AML. It is also tempting to hypothesize that the P450-mediated detoxification of drugs by the stroma is not a retinoid-specific phenomenon but rather a more general, cell-extrinsic mechanism of drug resistance.
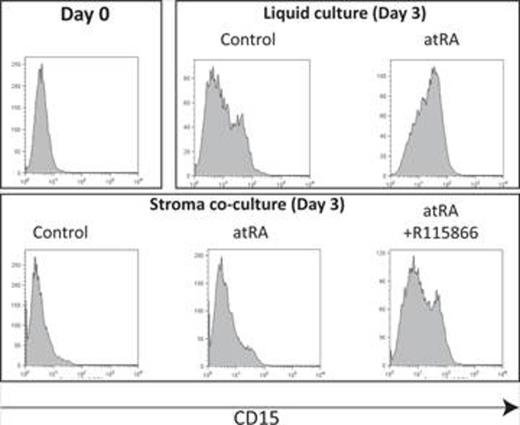
Flow cytometry analysis of CD15 expression of CD34+ blasts isolated from the BM of a patient with t(8;21) AML. On day 0, these cells express no CD15. Culture in the presence of serum results in rapid acquisition of CD15 (liquid control). This is further enhanced by treatment with 10-7M atRA. Co-culture with BM stroma inhibits CD15 expression and atRA has only minimal effect in the presence of stroma. Inhibition of stromal CYP26 overcomes stroma’s inhibition of atRA-mediated up-regulation of CD15.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal