Abstract
The cellular origin of human acute myeloid leukemia (AML) remains controversial. Limited G6PD-based X chromosome inactivation (XCI) studies suggested heterogeneity of leukemic stem cells (LSCs), with evidence of some AMLs resulting from multipotent CD34+/CD33- stem cells and others emerging from, or predominantly involving, lineage-committed CD34+/CD33+ myeloid precursors. So far, the difficulty of growing stem/progenitor cells from primary AML specimens has provided a major challenge for precise study of human LSCs and clinical implications of their heterogeneity. To overcome this, we developed a novel co-culture method to support long-term in vitro growth of primary AML specimens together with a highly sensitive XCI assay for isolated immature progenitor cells.
Diagnostic bone marrow specimens from 50 adult females with newly diagnosed AML were obtained from the SWOG Sample Repository. All patients received standard induction therapies without the CD33-targeting immunoconjugate, gemtuzumab ozogamicin. Human umbilical vein endothelial cells, transduced with a lentiviral construct encoding the open reading frame 1 of the early region 4 of adenovirus to provide growth factor/serum-independence, were co-cultured with aliquots of flow cytometrically-isolated CD34+/CD33- and CD34+/CD33+ cells in medium containing the aryl hydrocarbon receptor antagonist, StemReginin-1. Cultures were replenished with fresh medium weekly and kept in hypoxia for up to 8 weeks, and defined fractions plated after 4, 6, and 8 weeks for colony-forming cell assays. Resulting colony forming units-granulocyte and/or macrophage (CFU-GMs) were harvested and individually assessed for XCI via methylation-sensitive PCR-based analysis of a polymorphic short tandem repeat (STR) in the X-linked human androgen-receptor gene (HUMARA). Specimen growth was considered if it yielded ≥5 CFU-GMs after ≥4 weeks of co-culture. Specimens with >75% disease-specific CFU-GMs were considered clonal.
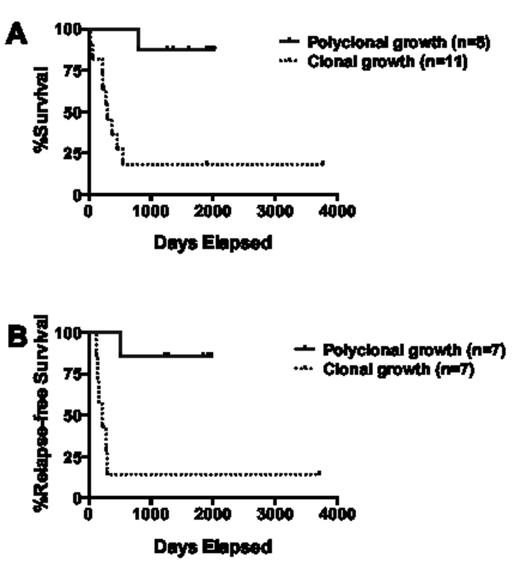
Among the 50 specimens, 45 (90%) were informative with regard to HUMARA STR, allowing clonal assessment of isolated cell progeny. Sixteen (35.6%) yielded CFU-GMs derived from CD34+/CD33- cells after long-term (≥4 weeks) co-culture sufficient for clonal analyses, whereas the remaining samples yielded either no growth (n=22) or insufficient CFU-GM growth (n=8) from this starting cell population. Five of the 16 specimens with sufficient CFU-GM growth derived from CD34+/CD33- cells (31.3%) yielded colonies consistent with a polyclonal growth pattern, and 3 samples without sufficient CFU-GM growth derived from CD34+/CD33- cells yielded colonies from unsorted cells that were consistent with a polyclonal growth pattern; these 8 leukemias were cytogenetically classified as favorable (n=3) or intermediate-risk (n=5, including 2 with normal karyotype and NPM1 but not FLT3/ITD mutation). In contrast, 11 specimens yielded CFU-GMs growth derived from CD34+/CD33- with a pattern consistent with clonal growth. The complete remission rate was slightly but statistically non-significantly higher among patients whose specimens yielded polyclonal CFU-GMs derived from CD34+/CD33- cells relative to those with clonal progenitor growth (87.5% vs. 63.6%, P=0.34). More importantly, however, the overall and relapse-free survival of patients whose specimens yielded polyclonal CFU-GMs derived from CD34+/CD33- or unsorted cells was significantly longer than that for patients whose specimens yielded a clonal progenitor growth pattern after long-term culture of CD34+/CD33- cells (P=0.0006 and P=0.0054, respectively; Figure 1); restriction of this analysis to the 16 specimens with sufficient CFU-GM growth from CD34+/CD33- cells did not change this outcome difference (e.g. hazard ratio for overall survival for restricted dataset: 0.13 [95% confidence interval: 0.06-0.72] vs 0.08 [0.04-0.44]).
Our novel co-culture method allows for the study of progenitor/stem cell involvement in a significant portion of human AMLs. Our data suggest that a subset of adult AMLs emerges from, or predominantly involves, only lineage-committed CD34+/CD33+ myeloid precursors but not the less mature CD34+/CD33- precursors. This subset, which is comprised of AMLs with diverse cytogenetic/molecular abnormalities, is characterized by an excellent outcome with intensive chemotherapy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal