Abstract
FLT3 is expressed in most human acute leukemias. When activated by FL, wild type (wt) FLT3 dimerizes and initiates downstream signals that result in proliferation and inhibition of apoptosis and differentiation. Activating FLT3 mutations (internal tandem duplications (ITDs) or point mutations) are common in AML and rare in ALL. ITD mutations confer a poor outcome in AML. In vitro, mutant FLT3 signaling can be further enhanced by binding of FL. Peripheral blood (PB) plasma FL levels rise in adults with AML, peaking about two weeks after initiation of chemotherapy. We sought to determine plasma levels of FL in pediatric patients after chemotherapy, and the functional effect of various levels of FL on both wt and mutant FLT3 leukemia cells.
FL levels were measured using FL ELISA on plasma samples (n=352) isolated from PB of children (n=75) enrolled on 4 multi-center acute leukemia clinical trials. Functional studies were performed on AML and ALL cell lines with wt FLT3 (HL60, RS4;11, SEMK2, and KOPN-8) and mutant FLT3 (MOLM14, MV4-11, and HB-1119). 72 hr etoposide IC50 was determined by WST-1 for each line. Cells were plated (250,000 cell/mL) for 72hr at etoposide IC50 in RPMI 1640 along with increasing concentrations of recombinant human FL (62.5 to 4,000 pg/ml). Cell cycle and apoptosis were analyzed using propidium iodide staining and annexin V/7-AAD binding, respectively. To explore the mechanism of FL effects, Ba/F3-ITD cells were incubated for 72hr in serum-free conditions with either 4,000 pg/mL (“high”), 62.5 pg/mL (“low”), or no FL. After washing, total and phosphorylated FLT3 protein levels were determined by Western blot.
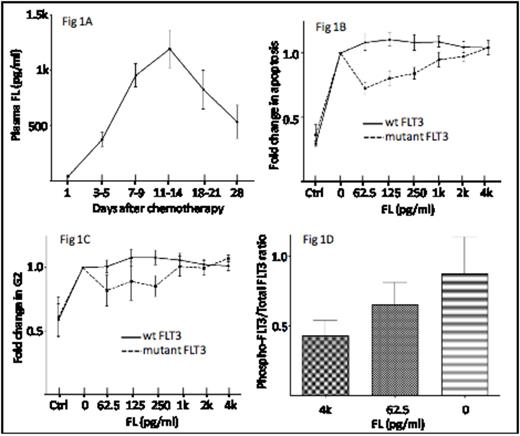
Pediatric patients receiving chemotherapy for the treatment of acute leukemia demonstrate a pattern of plasma FL rise with low levels at baseline (mean 41 pg/ml) and peak levels at day 11-14 following initiation of therapy (mean: 1,190 pg/mL; max: 5,783 pg/mL)(Fig 1A). Cell lines with FLT3 activating mutations selectively demonstrate resistance to etoposide-induced apoptosis (Fig 1B) and G2/M cell cycle arrest (Fig 1C) at low concentrations of FL (62.5 pg/mL). Dose-dependent reduction of etoposide resistance is seen with increasing concentrations of FL up to 4,000 pg/mL, suggesting that optimal etoposide-induced killing of FLT3-mutant leukemias may occur when FL plasma levels are at their peak. Ba/F3-ITD cells pre-incubated with peak concentrations of FL showed diminished baseline FLT3 phosphorylation, suggesting that the interaction of FL and FLT3/ITD exhibits substrate inhibition kinetics and results in a loss of FLT3/ITD-induced activation with high level FL exposure, thus providing a mechanistic basis for the observed loss of etoposide resistance.
Plasma FL rises to peak levels 11-14 days after initiation of chemotherapy. Through substrate inhibition of mutant FLT3 enzymatic activity, peak FL levels may reduce the etoposide resistance that characterizes FLT3-mutant leukemia cells exposed to pre-chemotherapy levels of FL. Thus, introduction of etoposide in a “time sequential” manner during periods of peak plasma FL levels may enhance killing of residual chemoresistant FLT3-mutant leukemia cells.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal