Abstract
Inhibitors are considered to be the most serious complication of clotting factor concentrate (CFC) therapy in hemophilia. The incidence of inhibitors in PTPs with severe hemophilia is very low (∼ 1 per 1,000 patient years). Four cases of PTP inhibitors that developed during hospital admissions were observed at the University of North Carolina over a 14-month period during 2011 – 2012. Due to concern about the possible role of the CFC used during hospitalization, further investigation by the CDC was requested.
A review of hemophilia-related hospital practices and procedures, along with a medical record review of admissions from January 2008 – November 2012 was conducted to: 1) compare inhibitor incidence trends in the time periods before and after January 2011; and 2) assess patient risk factors and evaluate hospital practices relative to inhibitor occurrence. A case was a person who developed an inhibitor during a hospital admission and non-cases were inpatients with no history of inhibitor development. The prevalence of inhibitor risk factors were compared between cases and non-cases and across time periods. Unadjusted odds ratios were used to compare the prevalence of exposures between cases and non-cases and comparisons of means of continuous risk factors used parametric and non-parametric tests as appropriate.
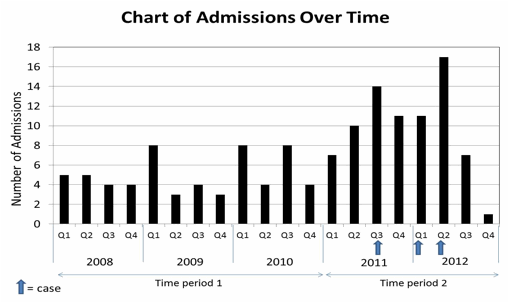
A check of the FDA's Adverse Event Reporting System (FAERS) revealed no identified problems of the CFC in question. The medical record review revealed 134 admissions in 49 patients (59 in period 1 and 75 in period 2) during the 5-year period (see Figure). No other cases were found during the review; however, one of the index cases was found to have developed the inhibitor as an outpatient leaving 3 cases and 45 non-cases for study. The cases ranged in age from 23 – 69 years. One had severe and one had moderate hemophilia A, while the third case had combined factor V-VIII deficiency with FV and FVIII levels of 7% and 10%, respectively. Compared to non-cases, cases had elevated odds of: an infection (OR, 95% CL=4.4, 0.3–53), continuous factor infusion (CI) (4.2, 0.04–6.4), at least one non-surgical procedure (3.9, 0.1–80.5), and used the only hospital-available CFC (2.7, 0.1–73) during the admission and a product switch prior to admission (3.5, 0.1–105) and a family history of hemophilia (2.4, 0.1–51). In addition, cases had more hospital admissions (mean 6.6 vs. 2.4, p = 0.002) and more total hospital days (mean 39.6 days vs. 14.8 days. p = 0.05) than non-cases. Finally, cases received a greater total dose of CFC than non-cases (mean 101 IU/kg vs. 69 IU/kg, p = 0.003) during admission.
Two hospital practices changed in period two: 1) the method of preparation and administration of CI involved less diluent and, therefore, a more concentrated infusion solution; and 2) the hospital pharmacy stocked only one brand of CFC in period 2 whereas several brands were available in period 1. The latter change increased the proportion of inpatients using a different product than that used as an outpatient (i.e., product switch) during period 2. Compared to inpatients admitted during period 1, those in period 2 were: more likely to receive the only available CFC and to have a product switch during the admission (p < 0.001). No difference between time periods was seen in the proportion of patients administered CI (58.8% vs. 56.9%) or who were tested for an inhibitor during admission (25% vs. 22%).
The investigation results support an increase in incidence of inhibitors among inpatients during period 2 compared to period 1, however, the number of cases was small. Nonetheless, because inhibitors are rare in PTPs, the occurrence of 3 cases in a 14-month period after a 3-year time span with no cases is highly suggestive of an actual increase in occurrence. The apparent increase was unlikely due to enhanced surveillance since rates of inhibitor testing during the two time periods were similar. While the odds ratios for some of the risk factors were elevated, the confidence limits were wide indicative of the lack of study power. Although there was no clear indication of a preventable inciting factor and more investigation is needed, the investigation revealed that this inhibitor cluster occurred in patients who had many complications that required lengthy hospitalizations and intense treatment with CFCs, contributing factors that should be considered in future management of hemophilia patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal