Abstract
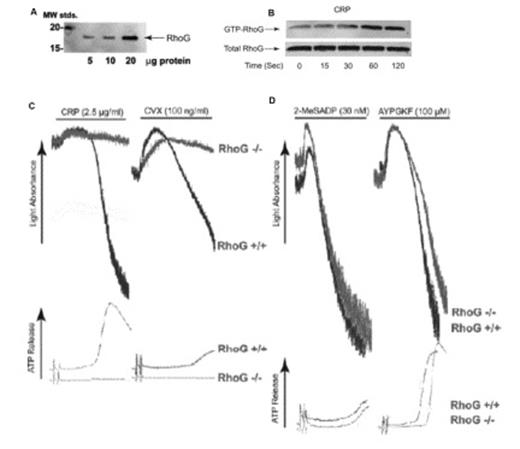
(A): Increasing amounts of human platelet lysate (in μg) were separated by SDS-PAGE, Western blotted, and probed with anti-RhoG antibody. (B) RhoG activation was measured upon stimulation of washed human platelets with 5μg/ml CRP for various times. Washed platelets were lysed and active GTP-bound RhoG was determined by pull-down analysis using bacterially expressed GST-ELMO. (C) Washed platelets from RhoG -/- mice and RhoG +/+ littermates were stimulated with GPVI agonists, 2.5 μg/ml CRP and 100 ng/ml convulxin and (D) G protein coupled receptor agonists, 30 nM 2MeSADP and 100 μM AYPGKF for 3.5 min under stirring conditions. Platelet aggregation and ATP secretion were measured by aggregometry.
(A): Increasing amounts of human platelet lysate (in μg) were separated by SDS-PAGE, Western blotted, and probed with anti-RhoG antibody. (B) RhoG activation was measured upon stimulation of washed human platelets with 5μg/ml CRP for various times. Washed platelets were lysed and active GTP-bound RhoG was determined by pull-down analysis using bacterially expressed GST-ELMO. (C) Washed platelets from RhoG -/- mice and RhoG +/+ littermates were stimulated with GPVI agonists, 2.5 μg/ml CRP and 100 ng/ml convulxin and (D) G protein coupled receptor agonists, 30 nM 2MeSADP and 100 μM AYPGKF for 3.5 min under stirring conditions. Platelet aggregation and ATP secretion were measured by aggregometry.
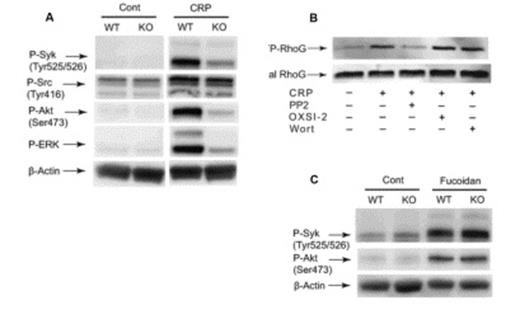
(A): Washed platelets from RhoG -/- mice and RhoG +/+ littermates were stimulated with 2.5 μg/ml CRP and at 37 °C for 2 min and probed with anti-phospho-Syk (Tyr525/526), anti-phospho-Src (Tyr416), anti-phospho-Akt (Ser473), anti-phospho-ERK, or anti-β-actin (lane loading control) antibodies by western blotting. (B): RhoG activation induced by 5μg/ml CRP for 60 sec was evaluated in the presence and absence of 10 μM PP2, 2 μM OXSI-2, or 100nM wortmannin. (C): Wild type and RhoG-deficient platelets were stimulated with 100 μg/ml fucoidan and probed with anti-phospho-Syk (Tyr525/526), anti-phospho-Akt (Ser473), or anti-β-actin (lane loading control) antibodies by western blotting.
(A): Washed platelets from RhoG -/- mice and RhoG +/+ littermates were stimulated with 2.5 μg/ml CRP and at 37 °C for 2 min and probed with anti-phospho-Syk (Tyr525/526), anti-phospho-Src (Tyr416), anti-phospho-Akt (Ser473), anti-phospho-ERK, or anti-β-actin (lane loading control) antibodies by western blotting. (B): RhoG activation induced by 5μg/ml CRP for 60 sec was evaluated in the presence and absence of 10 μM PP2, 2 μM OXSI-2, or 100nM wortmannin. (C): Wild type and RhoG-deficient platelets were stimulated with 100 μg/ml fucoidan and probed with anti-phospho-Syk (Tyr525/526), anti-phospho-Akt (Ser473), or anti-β-actin (lane loading control) antibodies by western blotting.
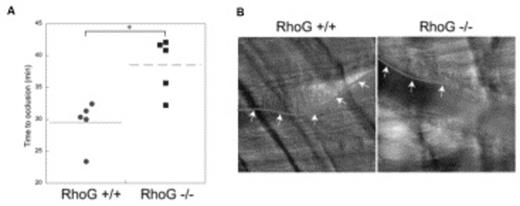
(A): Time required for occlusion of cremaster arterioles in RhoG +/+ and RhoG -/- mice was measured using microvascular thrombosis model with light/dye-induced injury. 5 mice of each genotype were used, and statistical analysis revealed a significant difference between the 2 genotypes of mice (*, P < .01). (B) Representative images of cremaster arterioles were taken from RhoG +/+ and RhoG -/- mice 30 min after the injury. As seen with the outline (arrows) of the thrombus formed, thrombus formation was inhibited in RhoG -/- mice.
(A): Time required for occlusion of cremaster arterioles in RhoG +/+ and RhoG -/- mice was measured using microvascular thrombosis model with light/dye-induced injury. 5 mice of each genotype were used, and statistical analysis revealed a significant difference between the 2 genotypes of mice (*, P < .01). (B) Representative images of cremaster arterioles were taken from RhoG +/+ and RhoG -/- mice 30 min after the injury. As seen with the outline (arrows) of the thrombus formed, thrombus formation was inhibited in RhoG -/- mice.
In conclusion, we show for the first time that RhoG is expressed and activated in platelets, plays an important role in GPVI/FcRγ-mediated platelet activation and is critical for thrombus formation in vivo.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal