Abstract
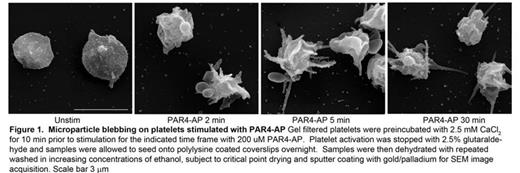
Microparticles are submicron lipid vesicles packed with protein and nucleic acid. They are found in abundance circulating in the vasculature and can be generated from a number of cell types including endothelial cells, leukocytes, and platelets. The microparticles generated from platelets far out number those generated from other cell types and their incidence is correlated with a myriad of cardiovascular diseases. We recently demonstrated that stimulation of gel filtered human platelets through Protease activated receptor (PAR) 4 leads to the generation of 4-5 times more platelet microparticles (PMP) than PAR1 stimulation. Platelet microparticle (PMP) production was demonstrated to be downstream of a Rho kinase-dependent signaling pathway. Consistently, more myosinIIa phosphorylation was observed downstream of PAR4 stimulation. PMP generation was quantified by flow cytometry using polystyrene microbeads of standardized size for appropriate size gating, and CD41a (aIIb) and CD62p (P-selectin) staining for positive identification of platelet derived membranes. Submicron particles positive for both CD41a and CD62p were classified as true PMP. Recently, we have expanded these observations to platelet stimulation with thrombin, convulxin (a GPVI collagen receptor agonist), and dual PAR/collagen receptor stimulation. Thrombin and PAR4-activating peptide (AP) stimulation leads to equivalent levels of PMP production, confirming that PAR4 is the major thrombin receptor responsible for PMP generation, with PAR1 playing only a minor role. Collagen receptor stimulation with convulxin lead to a comparable level of PMP generation as thrombin stimulation. However, co-stimulation with convulxin and thrombin or convulxin and PAR1-AP or PAR4-AP lead to PMP production exceeding the sum of PMP by convulxin and PAR agonist alone by as much as 350%, suggesting a synergistic response. It is well documented that PMP generation does not occur in the absence of extracellular Ca2+. Recently published data collected with Orai1 knockout mice indicate that the majority of extracellular Ca2+ entry into the platelet is mediated by the plasma membrane Ca2+ channel Orai1. STIM1 is an ER calcium sensor that migrates near the cell surface upon depletion of intracellular Ca2+ stores to oligomerize and activate Orai1. In an attempt to further elucidate the signaling pathways responsible for PMP generation we treated platelets with the STIM1 inhibitor SKF 96365. Preincubation with SKF 96365 nearly abolished PMP production induced by PAR4-AP, thrombin, convulxin, or the combination of PAR and collagen receptor agonist. Scanning Electron Microscopy of PAR4-AP stimulated platelets in suspension revealed extended filipodia and bag-like structures protruding from the platelet core which correlated with the size of PMPs as analyzed by flow cytometry. Pretreatment with the SKF 96365 or exclusion of extracellular Ca2+ prevented the formation of the microparticle-like extensions and blunted filipodia extension. Finally, confocal analysis of Orai1 staining on platelets spread on a collagen matrix and co-stimulated with PAR4-AP revealed Orai1 throughout the plasma membrane with intense staining of the microparticle-like structures. These data suggest that PMP generation is nucleated by STIM1 dependent Orai1 Ca2+ entry. Current efforts are focused on elucidating the mechanism by which PAR and collagen receptor agonists differentially regulate STIM1 or Orai1 activity to mediate PMP generation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal