Key Points
Protein kinase A (PKA) phosphorylates RhoA on serine188 to inhibit RhoA membrane translocation and RhoA kinase (ROCK) signaling.
Inhibition of RhoA/ROCK2 promotes myosin light chain (MLC) phosphatase activity, which prevents the phosphorylation of MLC and platelet shape change.
Abstract
Cyclic adenosine monophosphate (cAMP)-dependent signaling modulates platelet shape change through unknown mechanisms. We examined the effects of cAMP signaling on platelet contractile machinery. Prostaglandin E1 (PGE1)-mediated inhibition of thrombin-stimulated shape change was accompanied by diminished phosphorylation of myosin light chain (MLC). Since thrombin stimulates phospho-MLC through RhoA/Rho-associated, coiled-coil containing protein kinase (ROCK)-dependent inhibition of MLC phosphatase (MLCP), we examined the effects of cAMP on this pathway. Thrombin stimulated the membrane localization of RhoA and the formation of a signaling complex of RhoA/ROCK2/myosin phosphatase-targeting subunit 1 (MYPT1). This resulted in ROCK-mediated phosphorylation of MYPT1 on threonine 853 (thr853), the disassociation of the catalytic subunit protein phosphatase 1δ (PP1δ) from MYPT1 and inhibition of basal MLCP activity. Treatment of platelets with PGE1 prevented thrombin-induced phospho-MYPT1-thr853 in a protein kinase A (PKA)-dependent manner. Examination of the molecular mechanisms revealed that PGE1 induced the phosphorylation of RhoA on serine188 through a pathway requiring cAMP and PKA. This event inhibited the membrane relocalization of RhoA, prevented the association of RhoA with ROCK2 and MYPT1, attenuated the dissociation of PP1δ from MYPT1, and thereby restored basal MLCP activity leading to a decrease in phospho-MLC. These data reveal a new mechanism by which the cAMP-PKA signaling pathway regulates platelet function.
Introduction
Platelet shape change, which is critical for spreading and stable adhesion, requires the dynamic remodeling of the actin cytoskeleton. This is a complex temporal sequence driven by signaling events that regulate actin dynamics and by proteins that bind actin directly to facilitate its polymerization.1 The foremost regulator of actin function in platelets is myosin IIa, and the phosphorylation of the regulatory myosin light chains (MLCs) on serine19 (ser19) endows adenosinetriphosphatase activity that facilitates myosin interaction with actin filaments required for shape change and secretion of platelet granules.2 Increased intracellular Ca2+ in response to numerous platelet agonists activates Ca2+/calmodulin-dependent MLC kinase (MLCK), resulting in the phosphorylation of MLC-ser19, shape change, and secretion.3-8 Thrombin, thromboxane A2, and oxidized low-density lipoproteins (oxLDLs) also activate a Rho-associated, coiled-coil containing protein kinase (ROCK) pathway that drives the rearrangement of the actin cytoskeleton by inhibition of Ca2+-independent MLC phosphatase (MLCP).9-12 MLCP is composed of a 38-kDa protein phosphatase 1δ (PP1δ) catalytic subunit, a 130-kDa myosin phosphatase-targeting subunit 1 (MYPT1), and a 20-kDa subunit of unknown function.13 Activated ROCK forms a complex with MYPT1 leading to ROCK-mediated phosphorylation and inhibition of MLCP.9 Together with the activation of MLCK, the inhibition of MLCP promotes net phosphorylation of MLC required for platelet shape change, spreading, and thrombus stability.
The cyclic adenosine monophosphate (cAMP) pathway, activated by prostacyclin (PGI2), prostaglandin E1 (PGE1), and adenosine, inhibits multiple aspects of platelet function through protein kinase A (PKA), including reduced platelet aggregation in vitro and thrombosis in vivo.14-19 However, the precise molecular mechanism by which cAMP signaling inhibits platelet activatory signaling cascades remains unresolved. PKA phosphorylates Gα13, inositol trisphosphate receptor, vasodilator-stimulated phosphoprotein (VASP), actin-binding protein, and caldesmon, although the physiological relevance of the phosphorylation of these targets remains unclear.14 The identification of new substrates that are phosphorylated under physiological conditions and linked to the inhibition of specific platelet functions is required in order to understand the precise mechanisms that control unwanted platelet activity. With this in mind, we explored the mechanism underlying the ability of cAMP signaling to modulate the myosin contractile machinery that controls platelet shape change. We wanted to establish the effects of cAMP signaling on the RhoA/ROCK pathway in platelets and determine how this influenced the multimeric structure and activity of MLCP. Our study identifies RhoA as a novel target for cAMP/PKA signaling in platelets. cAMP signaling inhibits RhoA and ROCK activity to facilitate sustained activation of MLCP, which in turn prevents the phosphorylation of MLC required for platelet shape change. These findings provide evidence for a new mechanism of platelet regulation by cAMP signaling.
Methods
Reagents
These studies were approved by the Hull York Medical School Research Ethics Committee and conducted in accordance with the Declaration of Helsinki. The following antibodies were used; anti–phospho-VASP-ser157, anti-MYPT1, anti–phospho-MYPT1-thr853(threonine 853), anti-ROCK1 and ROCK2 (Cell Signaling Technology, Hitchin, UK); anti-PKAc antibody (BD Transduction Laboratories, Lexington, KY); anti–β-tubulin (Upstate Biotechnology, Dundee, UK); anti-PP1δ (Millipore, Watford, UK); anti-phospholipase Cγ2 (anti-PLCγ2), anti–integrin β3, and phospho-RhoA-ser188 (Santa Cruz Biotechnology, Calne, UK). Y27632, 1,2-bis-(o-aminophenoxy)ethane-tetra-acetic acid tetra-(acetoxymethyl) ester (BAPTA-AM), and GGTI-298 were from Calbiochem (Nottingham, UK). 8-(4-Chlorophenylthio)adenosine-3′,5′-cyclic monophosphorothioate Rp isomer (Rp-8-CPT-cAMPS), and 8-(4-chlorophenylthio)-N6-phenyladenosine-3′,5′-cyclic monophosphate (8-CPT-6-Phe-cAMP) were from Biolog (Breman, Germany). RhoA pull-down kit and Rho Activator I were from Cytoskeleton (Peterborough, UK). Para-nitrophenyl phosphate was from New England Biolabs (Hitchen, UK). All other reagents were obtained from Sigma Ltd (Poole, UK).
Platelet isolation
Human blood was taken from drug-free volunteers into acid citrate dextrose (29.9 mM Na3C6H5O7, 113.8 mM glucose, 72.6 mM NaCl, and 2.9 mM citric acid [pH 6.4]). Platelet-rich plasma was obtained by centrifugation of whole blood at 200g at 20°C for 20 minutes. Platelet-rich plasma was treated with citric acid (0.3 mM) and indomethacin (20 µM) and was centrifuged at 800g for 12 minutes. The platelet pellet was then suspended in wash buffer (36 mM citric acid, 10 mM EDTA, 5 mM glucose, 5 mM KCl, 9 mM NaCl) and centrifuged at 800g for 12 minutes. Platelets were resuspended (3 × 108 platelets per milliliter) in modified Tyrode’s buffer (150 mM NaCl, 5 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 0.55 mM NaH2PO4, 7 mM NaHCO3, 2.7 mM KCl, 0.5 mM MgCl2, 5.6 mM glucose [pH 7.4]) supplemented with apyrase (2 U/mL), indomethacin (10 μm), or EGTA (1 mm), unless otherwise stated.
Platelet shape change
LDL preparation and oxidation
LDL was prepared from fresh human plasma by sequential density ultracentrifugation and was oxidized as described previously (supplemental Methods).20
RhoA pull-down assay
Washed platelets (5 × 108 platelets per milliliter) were treated with agonists in the presence and absence of PGE1 at 37°C with stirring for 1 minute before stopping the reaction with an equal volume of lysis buffer. Lysates (300 µg) were incubated for 90 minutes at 4°C with Rhotekin-RBD beads (25 µg). Bead pellets were washed once, and Laemmli buffer was added prior to immunoblotting.
In vitro kinase assay
Recombinant human full-length active PKAcβ was incubated with recombinant human His-tagged RhoA (55 ng) in kinase buffer (25 mM 4-morpholinepropanesulfonic acid; pH 7.2], 12.5 mM glycerol-2-phosphate, 25 mM MgCl2, 5 mM EGTA, 2 mM EDTA, and 0.25 mM dithiothreitol) supplemented with adenosine triphosphate (400 µM) at 37°C for 15 minutes. Reaction was stopped by addition of Laemmli buffer to the mixture for further immunoblot analysis.
Measurement of cAMP
Washed platelets (2 × 108 platelets per milliliter) were treated with PGE1 (100 nM) for 1 minute in the presence and absence of inhibitors, and reactions were terminated by addition of lysis buffer. cAMP levels were assayed with a commercial enzyme immunoassay system and expressed as fmol cAMP per 1 × 107 platelets.21
Subcellular fractionation
Washed platelets (5 × 108 platelets per milliliter) were treated with agonists for the appropriate time, and reactions were stopped with an equal volume of fractionation buffer (320 mM sucrose, 4 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, and 0.5 mM Na3VO4 [pH 7.4]) supplemented with phosphatase and protease inhibitor cocktail.22 Samples were subjected to 5 freeze-thaw cycles. Intact platelets were removed by centrifugation at 5000g for 10 minutes at 4°C before centrifugation of lysates at 100 000g for 60 minutes at 4°C. The supernatant (soluble fraction) was removed, and the pellet (particulate fraction) was resuspended in lysis buffer. Protein concentrations were quantified by using a DC protein assay kit (Amersham Biosciences, UK). Equal protein concentrations of the fractions were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis and immunoblotted for PLCγ2 and integrin β3 to validate fractionation.
Immunoprecipitation and immunoblotting
Washed platelets (8 × 108 platelets per milliliter) were treated with agonists in the presence or absence of PGE1 and incubated at 37°C with stirring for the appropriate time. Reactions were stopped by the addition of an equal volume of ice-cold lysis buffer (150 mm NaCl, 10 mm tris(hydroxymethyl)aminomethane, 1 mm EDTA, 1 mm EGTA, 1% Igepal, 1 mm phenylmethanesulfonyl fluoride, 2.5 mm Na3VO4, phosphatase cocktail inhibitor, and protease cocktail inhibitor [pH 7.4]). Lysates were incubated overnight at 4°C with anti-RhoA (1 μg), anti-ROCK1 (1.5 μg), anti-ROCK2 (1.5 µg), anti-PP1δ (5 µg), or immunoglobulin G control antibodies, with constant rotation in the presence of protein A Sepharose beads. To prepare whole-cell lysates, platelets were then treated with the appropriate reagents before termination of the reactions with Laemmli buffer. Immunoprecipitates or lysates were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and analyzed by immunoblotting as previously described.21 The following primary antibodies were used; anti–phospho-VASP-ser157, anti–phospho-RhoA-ser188 (both 1:1000), anti-RhoA, anti-ROCK1, anti-ROCK2, anti-PP1δ (all 1:500), anti–phospho-MYPT1-thr853, and anti-MYPT1 (both 1:250).
Measurement of phosphatase activity
PP1δ was immunoprecipitated from unstimulated or thrombin-stimulated platelets in the presence or absence of PGE1 or Y27632. Immunoprecipitated PP1δ was incubated for 1 hour at room temperature with 5 mM para-nitrophenyl phosphate in reaction buffer (100 µL; 4 mM tris(hydroxymethyl)aminomethane-base, 2 mM KCl, 3 mM MgCl2, 0.1 mM MnCl2, 0.1 mg/mL bovine serum albumin, and 2 mM dithiothreitol [pH 8.1]). The reaction was stopped with 5 mM NaOH, and absorbance was measured at 405 nm.23 Specific PP1δ activity was obtained by subtracting the activity in the nonimmune immunoglobulin G immunoprecipitate from the activity in the PP1δ immunoprecipitate. To verify that each sample had equal amounts of PP1δ, samples were collected post-assay and immunoblotted for PP1δ.
Statistical analysis
Results are expressed as means ± standard error of the mean and were analyzed by using the Student t test or analysis of variance. The results were considered significant when P values were less than .05.
Results
PGE1 inhibits shape change and phosphorylation of MLC induced by thrombin
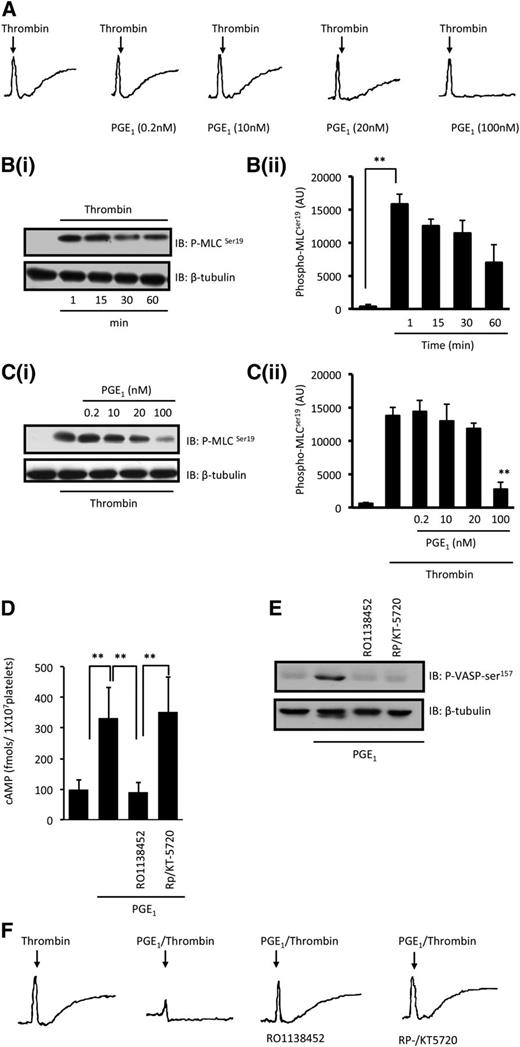
Under conditions that abrogated the effects of secondary signaling through adenosine 5′-diphosphate, thromboxane, and integrins, thrombin (0.05 U/mL) induced shape change, which was also associated with a rapid increase in phospho-MLC-ser19; phosphorylation was maximal at 1 minute and was maintained for up to 60 minutes (longest time tested) (Figure 1A-B). When platelets were treated with PGE1 (100 nM) prior to stimulation with thrombin, shape change was abolished and MLC phosphorylation was maintained at basal levels (Figure 1C). Confirmation that the cAMP/PKA signaling pathway was active under these conditions was evidenced by an increase in both cAMP from 98 ± 30 to 333 ± 101 fmols per 1 × 107 platelets (P < .05) (Figure 1D) and phospho-VASP-ser157 (Figure 1E), a marker of PKA signaling. The increased cAMP and phosphorylation of VASP-ser157 were ablated by the immunoprecipitate receptor antagonist RO1138452,24 while two structurally diverse inhibitors of PKA—Rp-8-CPT-cAMPS (500 µM)25 and KT-5720 (20 µM)—blocked only phospho-VASP-ser157. Importantly, these inhibitors also prevented PGE1 from inhibiting thrombin-induced shape change (Figure 1F). Consistent with these data, PGI2, which signals through cAMP, also inhibited thrombin-induced MLC phosphorylation (supplemental Figure 1). Thus, PGE1 (100 nM) abolished thrombin-induced shape change and MLC phosphorylation through a mechanism that may involve cAMP signaling.
PGE1 modulates thrombin-induced shape change and MLC phosphorylation. (A) Washed platelets (3 × 108 platelets per milliliter) were preincubated with apyrase (2 U/mL), indomethacin (10 µM), and EGTA (1 mM) followed by stimulation with thrombin (0.05 U/mL) in the presence and absence of PGE1 (0.2 to 100 nM), and traces were recorded for 2 minutes. Shown are representative traces of 3 separate experiments. (B) Washed platelets (3 × 108 platelets per milliliter) were stimulated with thrombin (0.05 U/mL) for the indicated time points and the levels of MLCser19 were assessed by immunoblotting. (i) Representative immunoblots (IB) from 3 independent experiments. (ii) Densitometric analysis of MLCser19 phosphorylation of 3 different experiments expressed as arbitrary units (AU). **P < .01 compared with basal levels. (C) Washed platelets (3 × 108 platelets per milliliter) were stimulated with thrombin (0.05 U/mL) for 1 minute in the presence or absence of PGE1 (0.2 to 100 nM) and phospho-MLCser19 was assessed by immunoblotting. (i) Representative immunoblots from 4 independent experiments. (ii) Densitometry of MLCser19 phosphorylation from 4 different experiments. **P < .01 compared with thrombin alone. (D) Washed platelets (2 × 108 platelets per milliliter) were treated with PGE1 (100 nM) in the presence and absence of RO1138452 (1 µM) or Rp-8-CPT-cAMPS (RP; 500 µM)/KT-5720 (20 µM), and cAMP levels were measured. Data are mean ± standard error of the mean of 3 experiments and are expressed as fmol cAMP/1 × 107 platelets. **P < .01 compared with basal levels. (E) Same as in (D), except platelets were lysed and immunoblotted for phospho-VASP-ser157. Blot is representative of 5 independent experiments. (F) Same as in (A), except that experiments were performed in the presence of RO1138452 (1 µM) or Rp-8-CPT-cAMPS (500 µM)/KT-5720 (20 µM). Shown are representative traces of 3 separate experiments.
PGE1 modulates thrombin-induced shape change and MLC phosphorylation. (A) Washed platelets (3 × 108 platelets per milliliter) were preincubated with apyrase (2 U/mL), indomethacin (10 µM), and EGTA (1 mM) followed by stimulation with thrombin (0.05 U/mL) in the presence and absence of PGE1 (0.2 to 100 nM), and traces were recorded for 2 minutes. Shown are representative traces of 3 separate experiments. (B) Washed platelets (3 × 108 platelets per milliliter) were stimulated with thrombin (0.05 U/mL) for the indicated time points and the levels of MLCser19 were assessed by immunoblotting. (i) Representative immunoblots (IB) from 3 independent experiments. (ii) Densitometric analysis of MLCser19 phosphorylation of 3 different experiments expressed as arbitrary units (AU). **P < .01 compared with basal levels. (C) Washed platelets (3 × 108 platelets per milliliter) were stimulated with thrombin (0.05 U/mL) for 1 minute in the presence or absence of PGE1 (0.2 to 100 nM) and phospho-MLCser19 was assessed by immunoblotting. (i) Representative immunoblots from 4 independent experiments. (ii) Densitometry of MLCser19 phosphorylation from 4 different experiments. **P < .01 compared with thrombin alone. (D) Washed platelets (2 × 108 platelets per milliliter) were treated with PGE1 (100 nM) in the presence and absence of RO1138452 (1 µM) or Rp-8-CPT-cAMPS (RP; 500 µM)/KT-5720 (20 µM), and cAMP levels were measured. Data are mean ± standard error of the mean of 3 experiments and are expressed as fmol cAMP/1 × 107 platelets. **P < .01 compared with basal levels. (E) Same as in (D), except platelets were lysed and immunoblotted for phospho-VASP-ser157. Blot is representative of 5 independent experiments. (F) Same as in (A), except that experiments were performed in the presence of RO1138452 (1 µM) or Rp-8-CPT-cAMPS (500 µM)/KT-5720 (20 µM). Shown are representative traces of 3 separate experiments.
cAMP signaling independently targets both Ca2+-dependent and RhoA/ROCK pathways
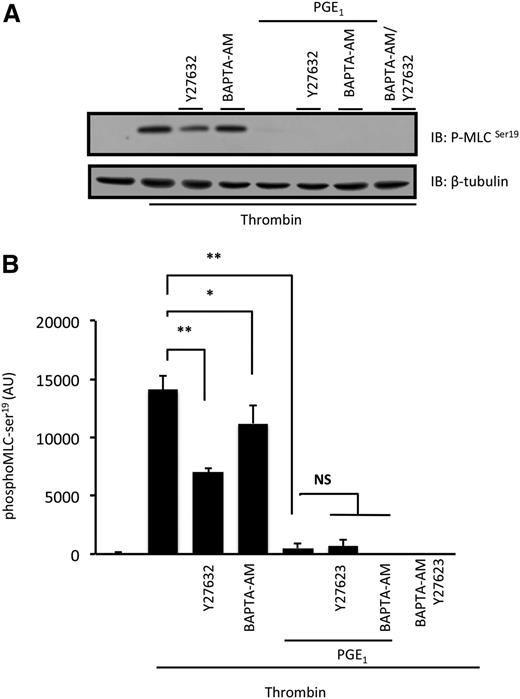
Thrombin stimulates the Ca2+-dependent activation of MLCK and RhoA-ROCK (Ca2+-independent) –dependent inhibition of MLCP in platelets to induce MLC phosphorylation.10,11 We examined whether PGE1 targeted these signaling cascades independently of each other by using BAPTA-AM to chelate intracellular Ca2+ and the widely used ROCK inhibitor Y27632 (10 µM).26,27 A combination of BAPTA-AM (20 µM) and Y27632 (10 µM) ablated thrombin-stimulated phospho-MLC-ser19, confirming a role for both pathways. Platelets were then preincubated with BAPTA-AM (20 µM) alone and stimulated with thrombin. We reasoned that under these conditions, only the ROCK pathway would be active, allowing examination of the effects of cAMP on ROCK signaling in isolation. BAPTA-AM reduced, but did not block, MLC phosphorylation. However, the combination of BAPTA-AM with PGE1 abolished the phosphorylation of MLC (Figure 2A), suggesting that ROCK signaling is blocked by cAMP. We next inhibited the ROCK pathway with Y27632 (10 µM) to study the effects of cAMP on the isolated Ca2+-dependent pathway stimulated by thrombin. Under these conditions, phospho-MLC-ser19 was reduced significantly but not blocked. The combination of Y27632 and PGE1 abolished phosphorylation of MLC-ser19 (Figure 2). Similar results were obtained with HA-1077, a structurally distinct ROCK inhibitor (data not shown). These data suggest that physiological activation of cAMP signaling can potentially target the RhoA/ROCK–dependent pathways independently of effects on Ca2+ mobilization to modulate platelet function.
PGE1 inhibits Ca2+ and RhoA/ROCK-dependent phosphorylation of MLC. Washed platelets (3 × 108 platelets per milliliter) were stimulated with thrombin (0.05 U/mL) for 1 minute in the presence or absence of Y27632 (10 µM), BAPTA-AM (20 µM), or PGE1 (100 nM), and phospho-MLCser19 was assessed by immunoblotting. (A) Representative immunoblots from 5 independent experiments. (B) Densitometry of MLCser19 phosphorylation of 5 different experiments. ns, not significant. *P < .05 compared with thrombin only; **P < .01 compared with thrombin only.
PGE1 inhibits Ca2+ and RhoA/ROCK-dependent phosphorylation of MLC. Washed platelets (3 × 108 platelets per milliliter) were stimulated with thrombin (0.05 U/mL) for 1 minute in the presence or absence of Y27632 (10 µM), BAPTA-AM (20 µM), or PGE1 (100 nM), and phospho-MLCser19 was assessed by immunoblotting. (A) Representative immunoblots from 5 independent experiments. (B) Densitometry of MLCser19 phosphorylation of 5 different experiments. ns, not significant. *P < .05 compared with thrombin only; **P < .01 compared with thrombin only.
PGE1 inhibits thrombin-induced activation of RhoA through phosphorylation of ser188
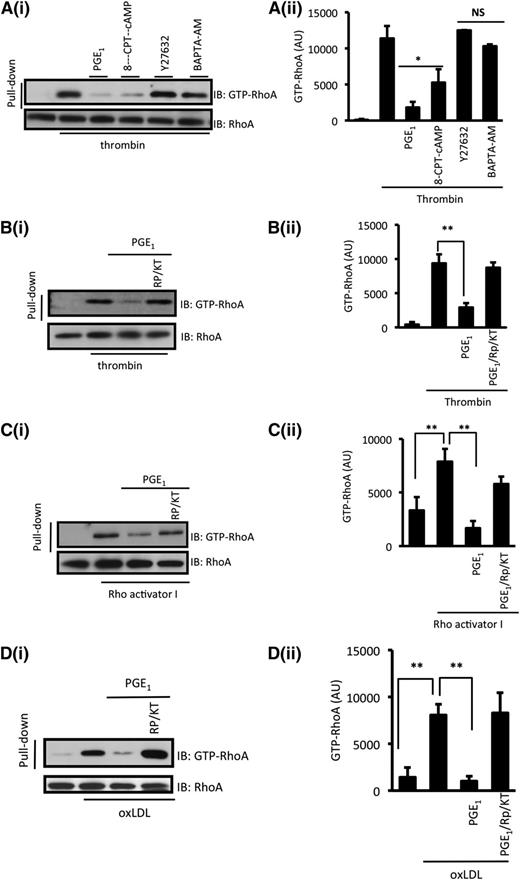
The pharmacologic inhibition of the ROCK and PKA pathways has limitations because of the potential off-target effects of kinase inhibitors.26,28 Therefore, to clarify the mechanism by which PGE1 regulated RhoA-ROCK–dependent signaling, we investigated activation of RhoA by using a guanosine triphosphate (GTP) –RhoA pull-down assay.29 Thrombin (0.05 U/mL) increased the levels of GTP-bound RhoA (Figure 3A), which was abolished if platelets were pretreated with PGE1 (100 nM). To confirm that our observation was cAMP mediated, we show that the cell-permeable nonhydrolyzable cAMP mimetic 8-CPT-6-Phe-cAMP (50 µM) and direct activator of PKA also inhibited formation of GTP-RhoA (Figure 3A). Inhibition of RhoA activation by PGE1 was prevented by the PKA inhibitor Rp-8-CPT-cAMPS and KT-5720 (Figure 3B), suggesting that PGE1 inhibits the activation of RhoA through a cAMP/PKA pathway. In contrast, Y27632 and BAPTA-AM had no effect on RhoA activation, indicating that cAMP signaling targets RhoA activation independently of any potential effects on ROCK activity and Ca2+ flux (Figure 3A).
cAMP/PKA signaling modulates the activation of RhoA. (A) Washed platelets (5 × 108 platelets per milliliter) were stimulated with thrombin (0.05 U/mL) for 1 minute in the presence or absence of Y27632 (10 µM), BAPTA-AM (20 µM), 8-CPT-6-Phe-cAMP (50 µM), or PGE1 (100 nM), and activated RhoA (GTP-RhoA) was assessed by using a pull-down assay with Rhotekin-RBD beads. (i) Representative immunoblots from 3 independent experiments. (ii) Densitometric analysis of activated RhoA (GTP-RhoA) of 3 different experiments. *P < .05 compared with thrombin only. (B) Same as in (A), except platelets were treated with PKA inhibitors Rp-8-CPT-cAMPS (500 µM)/KT-5720 (20 µM) for 15 minutes prior to addition of thrombin and PGE1. (C) Same as in (B), except platelets were treated with Rho Activator I (30 µM) for 2 minutes instead of thrombin. (D) Same as in (B), except platelets were treated with oxLDL (50 μg/mL) for 15 seconds instead of thrombin.
cAMP/PKA signaling modulates the activation of RhoA. (A) Washed platelets (5 × 108 platelets per milliliter) were stimulated with thrombin (0.05 U/mL) for 1 minute in the presence or absence of Y27632 (10 µM), BAPTA-AM (20 µM), 8-CPT-6-Phe-cAMP (50 µM), or PGE1 (100 nM), and activated RhoA (GTP-RhoA) was assessed by using a pull-down assay with Rhotekin-RBD beads. (i) Representative immunoblots from 3 independent experiments. (ii) Densitometric analysis of activated RhoA (GTP-RhoA) of 3 different experiments. *P < .05 compared with thrombin only. (B) Same as in (A), except platelets were treated with PKA inhibitors Rp-8-CPT-cAMPS (500 µM)/KT-5720 (20 µM) for 15 minutes prior to addition of thrombin and PGE1. (C) Same as in (B), except platelets were treated with Rho Activator I (30 µM) for 2 minutes instead of thrombin. (D) Same as in (B), except platelets were treated with oxLDL (50 μg/mL) for 15 seconds instead of thrombin.
PGI2 may induce phosphorylation of Gα12/13 to inhibit its activity.30 To confirm that cAMP targets RhoA independently of Gα12/13, we used two different approaches: Rho Activator I, which activates RhoA independently of G-protein coupled receptors (GPCRs),31,32 and oxLDL, which we have recently shown to activate RhoA downstream of CD36 and independently of GPCRs.12 Incubation of platelets with Rho Activator I (30 µM) and oxLDL (50 μg/mL) led to shape change (supplemental Figure 2), GTP loading, and activation of RhoA, which was inhibited by PGE1 (Figure 3C-D). The ability of PGE1 to block RhoA activation was prevented by Rp-8-CPT-cAMPS/KT-5720. Thus, PGE1 inhibits the activation of RhoA downstream of several independent pathways through a PKA-dependent mechanism.
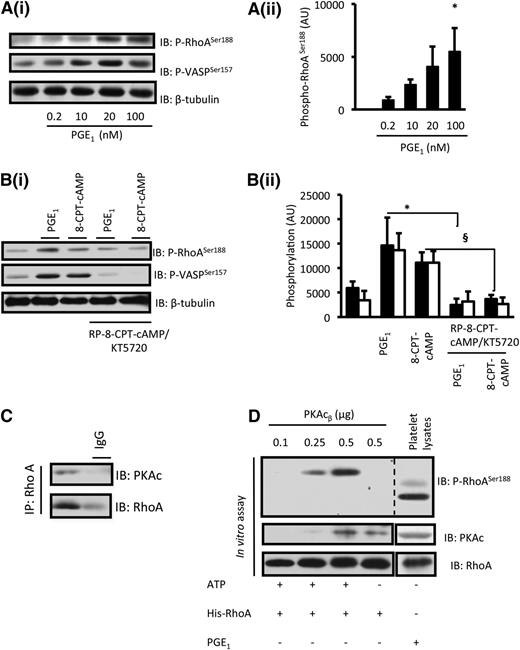
The phosphorylation of RhoA on ser188 may negatively regulate RhoA activity either through the inhibition of the GTPase or prevention of its membrane compartmentalization.33,34 Since PKA phosphorylates RhoA under in vitro conditions,28,35,36 we hypothesized that PKA-mediated phosphorylation of RhoA may account, at least in part, for the inhibition of RhoA by cAMP signaling. PGE1 (0.2 to 100 nM) induced a concentration-dependent increase in phospho-RhoA-ser188. Phosphorylation was maximal at 100 nM and was consistent in profile with the phosphorylation of VASP-ser157 (Figure 4A). Phosphorylation of RhoA in response to PGE1 occurred within 30 seconds and was maintained for 60 minutes (supplemental Figure 3). To determine whether phosphorylation of RhoA was mediated by PKA, we used a four-pronged strategy. First, PGE1-induced RhoA phosphorylation was reproduced by 8-CPT-6-Phe-cAMP (50 µM), which caused a significant phosphorylation of RhoA (Figure 4B). Second, Rp-8-CPT-cAMPS (500 µM)/KT-5720 (20 µM) prevented the phosphorylation of RhoA by both PGE1 (100 nM) and 8-CPT-6-Phe-cAMP (50 µM) (Figure 4B). Third, immunoprecipitation of RhoA from platelets demonstrated that the PKA catalytic subunit (PKAc) is associated with RhoA under basal conditions (Figure 4C), suggesting that RhoA could be a direct target for PKA in platelets. Fourth, to confirm that this association under physiological conditions could lead to the phosphorylation of RhoA by PKA, we performed an in vitro kinase assay. Incubation of recombinant human RhoA with recombinant active PKAc resulted in the phosphorylation of RhoA on ser188 (Figure 4D).
The phosphorylation of platelet RhoA in response to PGE1 is cAMP-PKA–dependent. (A) Washed platelets (3 × 108 platelets per milliliter) were incubated with PGE1 (0 to 100 nM) for 1 minute, and the phosphorylation of RhoA-ser188 and VASP-ser157 were determined by immunoblotting. (i) Representative immunoblots from 3 independent experiments. (ii) Densitometry for phospho-RhoA-ser188 from 3 different experiments. *P < .05 for untreated compared with PGE1 (100 nM). (B) Washed platelets (3 × 108 platelets per milliliter) were treated with PGE1 (100 nM) for 1 minute or 8-CPT-6-Phe-cAMP (50 µM) for 2 minutes in the presence or absence of Rp-8-CPT-cAMPS (500 µM)/KT-5720 (20 µM). RhoA-ser188 and VASP-ser157 phosphorylation were assessed by immunoblotting. (i) Representative immunoblots from 3 independent experiments. (ii) Densitometry for phospho-RhoA-ser188 (black bars) and VASP-ser157 (white bars) from 3 different experiments. *P < .05 PGE1 only compared with PGE1 with PKA inhibitors. §P < .05 8-CPT-6-Phe-cAMP compared with 8-CPT-6-Phe-cAMP with PKA inhibitors. (C) Washed platelets (8 × 108 platelets per milliliter) were lysed, and RhoA was immunoprecipitated from the lysate overnight. RhoA and PKAc in the immunoprecipitates (IP) were detected by immunoblotting. Representative blots for 3 experiments. (D) In lanes 1 to 4, recombinant active PKAcβ (0.1 to 0.5 µg) was incubated with recombinant His-tagged RhoA (55 ng) in the presence of adenosine triphosphate (ATP; 400 µM) for 15 minutes at 37°C. Reactions were stopped with 2× Laemmli buffer, and RhoAser188 phosphorylation was assessed by immunoblotting. In lane 4, ATP was omitted from the reaction mixture. In lane 5, whole-cell lysates from PGE1 (100 nM) –treated platelets were immunoblotted for phospho-RhoA-ser188. Shown are representative immunoblots from 3 independent experiments.
The phosphorylation of platelet RhoA in response to PGE1 is cAMP-PKA–dependent. (A) Washed platelets (3 × 108 platelets per milliliter) were incubated with PGE1 (0 to 100 nM) for 1 minute, and the phosphorylation of RhoA-ser188 and VASP-ser157 were determined by immunoblotting. (i) Representative immunoblots from 3 independent experiments. (ii) Densitometry for phospho-RhoA-ser188 from 3 different experiments. *P < .05 for untreated compared with PGE1 (100 nM). (B) Washed platelets (3 × 108 platelets per milliliter) were treated with PGE1 (100 nM) for 1 minute or 8-CPT-6-Phe-cAMP (50 µM) for 2 minutes in the presence or absence of Rp-8-CPT-cAMPS (500 µM)/KT-5720 (20 µM). RhoA-ser188 and VASP-ser157 phosphorylation were assessed by immunoblotting. (i) Representative immunoblots from 3 independent experiments. (ii) Densitometry for phospho-RhoA-ser188 (black bars) and VASP-ser157 (white bars) from 3 different experiments. *P < .05 PGE1 only compared with PGE1 with PKA inhibitors. §P < .05 8-CPT-6-Phe-cAMP compared with 8-CPT-6-Phe-cAMP with PKA inhibitors. (C) Washed platelets (8 × 108 platelets per milliliter) were lysed, and RhoA was immunoprecipitated from the lysate overnight. RhoA and PKAc in the immunoprecipitates (IP) were detected by immunoblotting. Representative blots for 3 experiments. (D) In lanes 1 to 4, recombinant active PKAcβ (0.1 to 0.5 µg) was incubated with recombinant His-tagged RhoA (55 ng) in the presence of adenosine triphosphate (ATP; 400 µM) for 15 minutes at 37°C. Reactions were stopped with 2× Laemmli buffer, and RhoAser188 phosphorylation was assessed by immunoblotting. In lane 4, ATP was omitted from the reaction mixture. In lane 5, whole-cell lysates from PGE1 (100 nM) –treated platelets were immunoblotted for phospho-RhoA-ser188. Shown are representative immunoblots from 3 independent experiments.
PGE1 inhibits membrane compartmentalization of RhoA
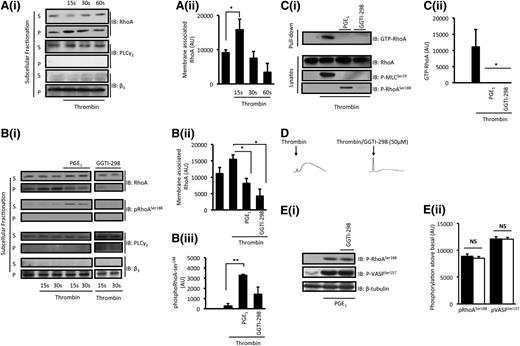
In unstimulated cells, RhoA is localized predominantly in the cytosol with a small fraction associated with the plasma membrane.35,37,38 We examined whether subcellular localization of RhoA was affected by PGE1. Small amounts of RhoA were observed in the particulate fraction with the majority in the soluble fraction. However, upon stimulation with thrombin (0.05 U/mL), there was a rapid translocation of RhoA to the particulate fraction, which peaked at 15 seconds before returning to basal levels at 60 seconds (Figure 5A). Preincubation with PGE1 (100 nM) ameliorated thrombin-induced RhoA membrane localization (Figure 5B). The inhibition of movement of RhoA to the membrane by PGE1 was mirrored by an increased phospho-RhoA-ser188 in the soluble fraction (Figure 5B). Similar effects were observed when platelets were stimulated with oxLDL (supplemental Figure 4), indicating that the inhibition of RhoA membrane localization was independent of GPCR-mediated platelet activation. Therefore, consistent with data on other cells,39,40 our data indicate that the phosphorylation of RhoA-ser188 in response to cAMP signaling may inhibit RhoA membrane compartmentalization.
Inhibition of thrombin-stimulated membrane localization of RhoA by PGE1. (A) Washed platelets (5 × 108 platelets per milliliter) were stimulated with thrombin for 15 to 60 seconds, and reactions were terminated by the addition of fractionation buffer and snap frozen. Samples were separated into soluble (S) and particulate (P) fractions by ultracentrifugation and were analyzed for the presence of RhoA, PLCγ2, and integrin β3 by immunoblotting. (i) Shown are representative blots of 5 independent experiments. (ii) Densitometry for RhoA in the particulate fraction from 5 different experiments. *P < .05 unstimulated compared with thrombin (15 seconds). (B) Washed platelets (5 × 108 platelets per milliliter) were stimulated with thrombin for 15 and 30 seconds in the presence and absence of PGE1 (100 nM) or GGTI-298 (50 µM). Soluble and particulate fractions were analyzed for the presence of RhoA, RhoA-ser188, PLCγ2, and integrin β3 by immunoblotting. (i) Representative blots of 4 independent experiments. (ii) Densitometry of the presence of RhoA in the particulate fractions at 15 seconds. *P < .05 thrombin compared with thrombin/PGE1 or thrombin/GGTI-298 (15 seconds). (iii) Densitometry of the presence of phospho-RhoAser188 in the soluble fraction at 15 seconds compared with untreated cells. **P < .01 thrombin compared with thrombin/PGE1. (C) Washed platelets (5 × 108 platelets per milliliter) were stimulated with thrombin (0.05 U/mL) for 1 minute in the presence and absence of PGE1 (100 nM) or GGTI-298 (50 µM), and reaction was stopped with lysis buffer. Lysates were incubated with Rhotekin-RBD beads (25 ng) for 90 minutes at 4°C. After washing the beads, the level of activated RhoA (GTP-RhoA) was assessed by immunoblotting. Lysates remaining after pull-down assay were used to examine phospho-RhoA-ser188, VASP-ser157, and MLC-ser19. (i) Representative immunoblots from 3 independent experiments. (ii) Densitometry of RhoA-GTP from 3 different experiments. *P < .05 thrombin alone compared with thrombin/PGE1 and GGTI-298. (D) Washed platelets (3 × 108 platelets per milliliter) were preincubated with apyrase (2 U/mL), indomethacin (10 µM), and EGTA (1 mM) followed by stimulation with thrombin (0.05 U/mL) in the presence and absence of GGTI-298 (50 μM), and traces were recorded for 2 minutes. Shown are representative traces of 3 separate experiments. (E) Washed platelets (5 × 108 platelets per milliliter) was treated with PGE1 (100 nM) for 1 minute in the presence (white bars) and absence (black bars) of GGTI-298 (50 µM), and the phosphorylation of RhoA and VASP was assessed by immunoblotting. (i) Representative immunoblots of phospho-RhoA-ser188 and phospho-VASP-ser157 from 3 independent experiments. (ii) Densitometry of phosphorylation of RhoA from 3 different experiments. NS, not significant.
Inhibition of thrombin-stimulated membrane localization of RhoA by PGE1. (A) Washed platelets (5 × 108 platelets per milliliter) were stimulated with thrombin for 15 to 60 seconds, and reactions were terminated by the addition of fractionation buffer and snap frozen. Samples were separated into soluble (S) and particulate (P) fractions by ultracentrifugation and were analyzed for the presence of RhoA, PLCγ2, and integrin β3 by immunoblotting. (i) Shown are representative blots of 5 independent experiments. (ii) Densitometry for RhoA in the particulate fraction from 5 different experiments. *P < .05 unstimulated compared with thrombin (15 seconds). (B) Washed platelets (5 × 108 platelets per milliliter) were stimulated with thrombin for 15 and 30 seconds in the presence and absence of PGE1 (100 nM) or GGTI-298 (50 µM). Soluble and particulate fractions were analyzed for the presence of RhoA, RhoA-ser188, PLCγ2, and integrin β3 by immunoblotting. (i) Representative blots of 4 independent experiments. (ii) Densitometry of the presence of RhoA in the particulate fractions at 15 seconds. *P < .05 thrombin compared with thrombin/PGE1 or thrombin/GGTI-298 (15 seconds). (iii) Densitometry of the presence of phospho-RhoAser188 in the soluble fraction at 15 seconds compared with untreated cells. **P < .01 thrombin compared with thrombin/PGE1. (C) Washed platelets (5 × 108 platelets per milliliter) were stimulated with thrombin (0.05 U/mL) for 1 minute in the presence and absence of PGE1 (100 nM) or GGTI-298 (50 µM), and reaction was stopped with lysis buffer. Lysates were incubated with Rhotekin-RBD beads (25 ng) for 90 minutes at 4°C. After washing the beads, the level of activated RhoA (GTP-RhoA) was assessed by immunoblotting. Lysates remaining after pull-down assay were used to examine phospho-RhoA-ser188, VASP-ser157, and MLC-ser19. (i) Representative immunoblots from 3 independent experiments. (ii) Densitometry of RhoA-GTP from 3 different experiments. *P < .05 thrombin alone compared with thrombin/PGE1 and GGTI-298. (D) Washed platelets (3 × 108 platelets per milliliter) were preincubated with apyrase (2 U/mL), indomethacin (10 µM), and EGTA (1 mM) followed by stimulation with thrombin (0.05 U/mL) in the presence and absence of GGTI-298 (50 μM), and traces were recorded for 2 minutes. Shown are representative traces of 3 separate experiments. (E) Washed platelets (5 × 108 platelets per milliliter) was treated with PGE1 (100 nM) for 1 minute in the presence (white bars) and absence (black bars) of GGTI-298 (50 µM), and the phosphorylation of RhoA and VASP was assessed by immunoblotting. (i) Representative immunoblots of phospho-RhoA-ser188 and phospho-VASP-ser157 from 3 independent experiments. (ii) Densitometry of phosphorylation of RhoA from 3 different experiments. NS, not significant.
To confirm that membrane compartmentalization of RhoA was critical for its activation, we used an inhibitor of protein geranylgeranyltransferase type I (GGTase I), GGTase I inhibitor 298 (GGTI-298), which prevents the association of RhoA and Ras with the plasma membrane through inhibition of protein isoprenylation.41 If translocation of RhoA preceded its activation, then GGTI-298 would inhibit both translocation and GTP loading. Conversely, if the loading of GTP occurs prior to membrane localization, then GGTI-298 would inhibit only RhoA translocation but not GTP loading. Pretreatment of platelets with GGTI-298 (50 µM) inhibited RhoA membrane translocation, GTP loading of RhoA, and shape change in response to thrombin (Figure 5B-D). To confirm that cAMP-mediated phosphorylation of RhoA occurred in the cytosol, we examined phospho-RhoA-ser188 in the presence of GGTI-298. PGE1-induced VASP-ser157 and RhoA-ser188 phosphorylation were unaffected by GGTI-298, suggesting that phospho-RhoA-ser188 is generated in the cytosolic fraction and that the inhibition of isoprenylation has no effect on activation of the cAMP signaling cascade (Figure 5E). These data demonstrate that PGE1 prevents RhoA activation by blocking its membrane translocation, potentially through phosphorylation of RhoA on ser188.
PGE1 inhibits formation of RhoA/ROCK2/MYPT1 multiprotein complex
ROCK is a downstream effector of RhoA that is proposed to drive platelet shape change and secretion through inhibition of MLCP.9,11 Having established that cAMP signaling phosphorylated and inhibited RhoA, we wanted to determine the downstream consequences. ROCK activation leads to the phosphorylation of MYPT1 on thr696 and thr853, the targeting subunit of MLCP, which inhibits the phosphatase.13 We used the phosphorylation of the best-characterized site—thr85—as a marker of ROCK activity. Thrombin-induced phospho-MYPT1-thr853 was abolished by Y27632 but not by BAPTA-AM (Figure 6A), confirming the role of ROCK. PGE1 (100 nM) blocked thrombin-stimulated inhibitory phosphorylation of MYPT1-thr853 (Figure 6B) which, in turn, was prevented by inhibition of PKA (Figure 6C). To confirm that membrane localization of RhoA is required for ROCK-mediated phospho-MYPT1-thr853, we repeated these experiments in the presence of GGTI-298. Under these conditions, thrombin stimulation of phospho-MYPT1-thr853 was abolished (Figure 6B). In addition, both Rho Activator I and oxLDL induced the phosphorylation of MYPT1-thr853, which was prevented by the presence of PGE1 (Figure 6D-E) and was associated with phosphorylation of RhoA-ser188. Together, these data suggest that inhibition of RhoA by cAMP signaling prevents the activation of ROCK and phosphorylation of MLCP, regardless of the mechanism of RhoA activation.
PGE1 modulates the formation of a RhoA/ROCK/MYPT1 signaling complex. (A) Washed platelets (5 × 108 platelets per milliliter) were stimulated with thrombin (0.05 U/mL) for 1 minute in the presence or absence of Y27632 (10 µM) and BAPTA-AM (20 µM); phospho-MYPT1-thr853 was then assessed by immunoblotting. (i) Representative immunoblots from 3 independent experiments. (ii) Densitometry of phospho-MYPT1-thr853 from 3 different experiments. **P < .01 thrombin compared with basal levels; §P < .05 thrombin alone compared with Y27632. (B) Same as in (A), except platelets were treated with PGE1 (100 nM) or GGTI-298 (50 µM) prior to stimulation with thrombin, and phospho-MYPT1-thr853 was assessed by immunoblotting. (i) Representative immunoblots from 3 independent experiments. (ii) Densitometry of MYPT1-thr853 phosphorylation of 3 different experiments. **P < .01 thrombin alone compared with thrombin/PGE1 and GGTI-298. (C) Washed platelets (5 × 108 platelets per milliliter) were stimulated with thrombin (0.05 U/mL) for 1 minute in the presence or absence of PGE1 (100 nM), and phospho-MYPT1-thr853 was assessed by immunoblotting. In some cases, platelets were also treated with Rp-8-CPT-cAMPS (500 µM)/KT-5720 (20 µM). (i) Representative immunoblots from 3 independent experiments. (ii) Densitometric analysis of phospho-MYPT1thr853 of 3 different experiments. *P < .05 compared with thrombin only. (D) Same as in (B), except platelets were treated with Rho Activator I (30 µM) for 2 minutes instead of thrombin. (E) Same as in (B), except platelets were treated with oxLDL (50 μg/mL) for 15 seconds instead of thrombin. (F) Washed platelets (8 × 108 platelets per milliliter) were stimulated with thrombin (0.05 U/mL) for 1 minute in the presence and absence of PGE1 (100 nM). Reaction was stopped with lysis buffer and ROCK1 was immunoprecipitated. The immunoprecipitates were then immunoblotted for the presence of ROCK2, MYPT1, and RhoA. (i) Representative immunoblots of 4 independent experiments. (ii) Densitometric analysis of amount of RhoA present in the immunoprecipitates. *P < .05 thrombin compared with thrombin alone. (G) Same as in (F), except PP1δ was immunoprecipitated and was incubated for 1 hour at room temperature with 5 mM para-nitrophenyl phosphate, and absorbance was measured at 405 nm. Graph is representative of 5 different experiments. **P < .01 thrombin compared with basal activity; *P < .05 thrombin alone compared with thrombin with PGE1. (H) Samples from (G) were immunoblotted for PP1δ and MYPT1. (i) Representative immunoblots of 4 independent experiments. (ii) Densitometric analysis of total MYPT1 of 4 different experiments. *P < .05 thrombin compared with basal levels; §P < .05 thrombin alone compared with thrombin with PGE1.
PGE1 modulates the formation of a RhoA/ROCK/MYPT1 signaling complex. (A) Washed platelets (5 × 108 platelets per milliliter) were stimulated with thrombin (0.05 U/mL) for 1 minute in the presence or absence of Y27632 (10 µM) and BAPTA-AM (20 µM); phospho-MYPT1-thr853 was then assessed by immunoblotting. (i) Representative immunoblots from 3 independent experiments. (ii) Densitometry of phospho-MYPT1-thr853 from 3 different experiments. **P < .01 thrombin compared with basal levels; §P < .05 thrombin alone compared with Y27632. (B) Same as in (A), except platelets were treated with PGE1 (100 nM) or GGTI-298 (50 µM) prior to stimulation with thrombin, and phospho-MYPT1-thr853 was assessed by immunoblotting. (i) Representative immunoblots from 3 independent experiments. (ii) Densitometry of MYPT1-thr853 phosphorylation of 3 different experiments. **P < .01 thrombin alone compared with thrombin/PGE1 and GGTI-298. (C) Washed platelets (5 × 108 platelets per milliliter) were stimulated with thrombin (0.05 U/mL) for 1 minute in the presence or absence of PGE1 (100 nM), and phospho-MYPT1-thr853 was assessed by immunoblotting. In some cases, platelets were also treated with Rp-8-CPT-cAMPS (500 µM)/KT-5720 (20 µM). (i) Representative immunoblots from 3 independent experiments. (ii) Densitometric analysis of phospho-MYPT1thr853 of 3 different experiments. *P < .05 compared with thrombin only. (D) Same as in (B), except platelets were treated with Rho Activator I (30 µM) for 2 minutes instead of thrombin. (E) Same as in (B), except platelets were treated with oxLDL (50 μg/mL) for 15 seconds instead of thrombin. (F) Washed platelets (8 × 108 platelets per milliliter) were stimulated with thrombin (0.05 U/mL) for 1 minute in the presence and absence of PGE1 (100 nM). Reaction was stopped with lysis buffer and ROCK1 was immunoprecipitated. The immunoprecipitates were then immunoblotted for the presence of ROCK2, MYPT1, and RhoA. (i) Representative immunoblots of 4 independent experiments. (ii) Densitometric analysis of amount of RhoA present in the immunoprecipitates. *P < .05 thrombin compared with thrombin alone. (G) Same as in (F), except PP1δ was immunoprecipitated and was incubated for 1 hour at room temperature with 5 mM para-nitrophenyl phosphate, and absorbance was measured at 405 nm. Graph is representative of 5 different experiments. **P < .01 thrombin compared with basal activity; *P < .05 thrombin alone compared with thrombin with PGE1. (H) Samples from (G) were immunoblotted for PP1δ and MYPT1. (i) Representative immunoblots of 4 independent experiments. (ii) Densitometric analysis of total MYPT1 of 4 different experiments. *P < .05 thrombin compared with basal levels; §P < .05 thrombin alone compared with thrombin with PGE1.
The phosphorylation and inhibition of MLCP by ROCK requires the formation of a multienzyme complex of RhoA, ROCK, and MLCP.42 However, the consequence of RhoA phosphorylation by cAMP/PKA and identity of the ROCK isoform present in this key complex is unclear. By using isoform-specific antibodies and immunoprecipitation, we detected both ROCK1 and ROCK2 in platelet lysates (supplemental Figure 5).43 Consistent with studies in smooth muscle cells,44 co-immunoprecipitation studies demonstrated that under basal conditions, RhoA was associated with ROCK2, but the amount of RhoA associated with ROCK2 increased in response to thrombin. However, in the presence of PGE1 (100 nM), the association of RhoA/ROCK2 was similar to that observed in resting platelets (Figure 6F). We next examined the interaction between ROCK2 and MYPT1. We found no evidence of association between ROCK2 and MYPT1 under basal conditions; we did find that MYPT1 was recruited to the RhoA/ROCK2 complex in response to thrombin. PGE1 attenuated the thrombin-stimulated association of these proteins (Figure 6F). To confirm that the inhibition of ROCK/MYPT1 influenced MLCP activity, we used an anti-PP1δ–specific antibody to immunoprecipitate the phosphatase.9 The basal activity of myosin phosphatase was reduced significantly by stimulation with thrombin (1 ± 0 to 0.3 ± 0.1; P < .05). Pretreatment with PGE1 prevented the inhibition of MLCP and reversed the activity to basal levels (1 ± 0.1) (Figure 6G). In ferret portal smooth muscle cells, the loss of PPIδ activity in response to cell activation is caused by its disassociation from MYPT1.45 Consistent with those observations, we found that under basal conditions, PP1δ is associated with MYPT1 (Figure 6H). However, stimulation with thrombin led to the disassociation of this complex. When platelets were treated with PGE1, the ability of thrombin to cause the disassociation was lost, and inhibition of MLCP activity was prevented.
Discussion
The cAMP-PKA signaling cascade modulates a number of important platelet functions including Ca2+ mobilization and granule secretion and aggregation.15,17,18 An early study indicated that cAMP signaling inhibited platelets independently of its effects on Ca2+ mobilization,15 although the precise mechanisms remain unclear. Here, we present evidence for a new mechanism by which cAMP regulates platelet function. Our novel findings are that (1) cAMP signaling inhibits platelet shape change and MLC phosphorylation through activation of MLCP, (2) the activation of MLCP occurs through cAMP-mediated phosphorylation and inhibition of RhoA and downstream ROCK activity, and (3) cAMP prevents the formation of a multienzyme complex of RhoA/ROCK2/MYPT1 and promotes association of MYPT1 with the catalytic subunit PP1δ.
A recent study showing that RhoA depletion results in platelets with altered shape and function46 has placed renewed importance on understanding the regulation of this small G-protein. In the absence of the availability of RhoA-deficient mice, we used a less stringent pharmacologic isolation of ROCK signaling to show that cAMP targets this pathway independently of any effect on Ca2+ signaling. Y27632 targets the catalytic adenosinetriphosphatase domain of ROCK, which shares homology with other serine-threonine kinases and therefore may not be entirely specific.26,28 Since using this pharmacologic approach leaves open the possibility that cAMP targets other kinases to regulate shape change, we used a pull-down assay to demonstrate that cAMP signaling prevents the activation of platelet RhoA upstream of ROCK. In this regard, cAMP signaling may target the activation of platelet Gα12/13 to prevent RhoA activation,30,47 although this remains unconfirmed in other cell types. We found that the activation of cAMP signaling or PKA directly blocks RhoA activation and ROCK signaling in response to Gα12/13-dependent, receptor-independent, and CD36-mediated stimulation, suggesting that the actions of cAMP on RhoA are independent of its mode of activation. Moreover, we provide multiple lines of evidence to suggest that RhoA may be a novel physiological target of cAMP-PKA signaling in platelets. PGE1 and the cAMP mimetic 8-CPT-6-Phe-cAMP induce rapid and sustained phosphorylation of RhoA-ser188, which is blocked by two structurally distinct inhibitors of PKA. The possibility that RhoA is a physiological substrate for PKA signaling is strengthened by observations demonstrating that RhoA is phosphorylated by PKA in vitro and that the catalytic subunit of PKA associates with RhoA in vivo, which could facilitate a phosphorylation event similar to those observed in other cells.35 In lymphocytes, fibroblasts, and smooth muscle cells, phosphorylation at ser188 by PKA or expression of a phosphomimetic RhoA-ser188 (RhoA-188A) prevents RhoA movement to the membrane.35,48 Consistent with the results of these studies, the results of our study showed that the thrombin-induced membrane compartmentalization of platelet RhoA was prevented by PGE1 and was associated with increased cytosolic phospho-RhoA-ser188. The cytosolic compartmentalization of RhoA by the pharmacologic inhibition of isoprenylation also resulted in maintenance of the guanosine diphosphate–bound form. Thus, the ability of cAMP signaling to prevent membrane relocalization of RhoA likely reduces its activation, potentially through guanosine diphosphate dissociation inhibitors (GDIs), since phospho-RhoA-ser188 increases its interaction with GDIs and enhances the ability of GDIs to extract RhoA from membranes.33,34
RhoA/ROCK signaling induced by thrombin inactivates MLCP, leading to phosphorylation of MLC and platelet shape change.49-51 By using co-immunoprecipitation, we showed for the first time that platelet activation by thrombin caused formation of a multienzyme RhoA/ROCK2/MYPT1 complex,52 which led to the inhibitory phosphorylation of MYPT1. Moreover, cAMP signaling prevents the formation of the RhoA/ROCK2 complex and, under these conditions, ROCK2 cannot associate with MYPT1 (Figure 7). By using a site-specific antibody to phospho-MYPT1-thr853, we demonstrated that cAMP prevents thrombin-stimulated inhibitory phosphorylation of MLCP. Since inhibition of membrane localization of RhoA by blocking its isoprenylation also inhibited thrombin-stimulated phospho-MYPT1-thr853, our data suggest that the cytosolic compartmentalization of RhoA by cAMP signaling prevents the formation of the multienzyme RhoA/ROCK2/MYPT1 complex required to inhibit MLCP. These data are consistent with observations in smooth muscle cells in which cAMP-mediated inactivation of RhoA leads to activation of MLCP.48 In smooth muscle cells, PKA and protein kinase G prevent or reverse agonist-mediated inhibitory phosphorylation on thr696 and thr853 and depression of MLCP activity.49,53 We demonstrate that an analogous mechanism exists in platelets. Thrombin causes a significant depression in platelet PP1δ activity, which is associated with the disassociation of PP1δ from MYPT1, a phenomenon already reported in smooth muscle cells.45 cAMP caused a modest increase in basal PPIδ activity but completely reversed the inhibitory effects of thrombin on PP1δ activity. It seems that a major role of cAMP signaling is to prevent this dissociation of PP1δ from MYPT1 and maintain the holoenzyme complex and phosphatase activity. Our data suggest that one mechanism to achieve this is the inhibition of RhoA/ROCK signaling. In smooth muscle cells,49 cAMP signaling targets MYPT1 directly to influence phosphatase activity, and it is therefore possible that regulation of RhoA represents only one aspect of MLCP regulation by cAMP signaling.
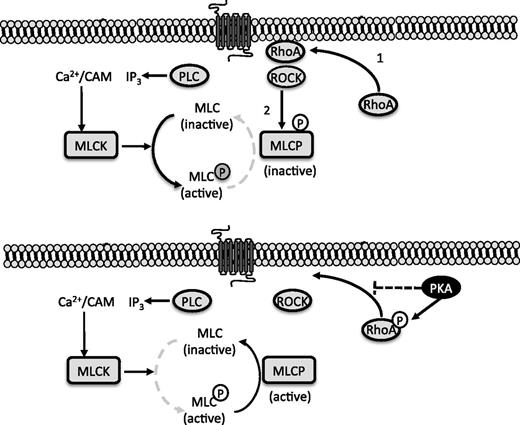
cAMP signaling caused activation of MLCP through regulation of RhoA/ROCK. Diagrammatic representation of the cross talk between cAMP and RhoA/ROCK signaling in platelets. Platelet activation leads to membrane relocalization and GTP loading of RhoA, which facilitates the activation of ROCK (1). A RhoA/ROCK/MYPT1 complex is formed leading to ROCK-dependent inhibitory phosphorylation of MLCP (2). Simultaneously, phospholipase C (PLC) is activated to generate inositol trisphosphate (IP3) and release intracellular Ca2+. The increased Ca2+ concentrations leads to the calmodulin (CAM) dependent activation of MCLK. Under these conditions, the ability of MLCP to dephosphorylate MLC is diminished (gray dotted line; upper panel). Activated PKA phosphorylates RhoA on ser188, which prevents membrane relocalization and GTP loading. ROCK-mediated phosphorylation of MYPT1 is prevented, leaving MLCP in its active state. Here the dephosphorylation of MLC is the predominant pathway, while phosphorylation of MLC by MLCK is diminished (lower panel).
cAMP signaling caused activation of MLCP through regulation of RhoA/ROCK. Diagrammatic representation of the cross talk between cAMP and RhoA/ROCK signaling in platelets. Platelet activation leads to membrane relocalization and GTP loading of RhoA, which facilitates the activation of ROCK (1). A RhoA/ROCK/MYPT1 complex is formed leading to ROCK-dependent inhibitory phosphorylation of MLCP (2). Simultaneously, phospholipase C (PLC) is activated to generate inositol trisphosphate (IP3) and release intracellular Ca2+. The increased Ca2+ concentrations leads to the calmodulin (CAM) dependent activation of MCLK. Under these conditions, the ability of MLCP to dephosphorylate MLC is diminished (gray dotted line; upper panel). Activated PKA phosphorylates RhoA on ser188, which prevents membrane relocalization and GTP loading. ROCK-mediated phosphorylation of MYPT1 is prevented, leaving MLCP in its active state. Here the dephosphorylation of MLC is the predominant pathway, while phosphorylation of MLC by MLCK is diminished (lower panel).
The inhibitory effects of cAMP/PKA on RhoA signaling provide a new mechanism through which endothelial-derived platelet mediators could modulate platelet shape change. The inactivation of RhoA by cAMP/PKA prevents the formation of a RhoA/ROCK2 signaling complex, which attenuates the inhibitory phosphorylation of MLCP and prevents the disassociation of PP1δ/MYPT1 complex. Under these conditions, the phosphatase could remain active, thereby preventing phosphorylation of MLC and inhibiting shape change (Figure 7). The use of protein kinase inhibitors has allowed us to delineate a potentially new pathway controlling platelet shape change. However, given the complexity of signaling pathway and potential off-target effects of the inhibitors we have used, the generation of conditional knockouts for platelet-selective deletion of ROCK1, ROCK2, and PKA are required to confirm the regulation of MLCP by ROCK2 and the ability of PKA to modulate ROCK2 activity. In conclusion, the discovery of RhoA as a novel substrate of cAMP signaling and targeting of the RhoA/ROCK2/MLCP pathway represents a new and important aspect of the mechanism by which cAMP modulates platelet function.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by The British Heart Foundation (PG/10/90/28636).
Authorship
Contribution: A.A. performed experiments and analyzed and interpreted data; K.S.W., Z.R., R.L., and S.M. performed experiments; and K.M.N. designed research, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Khalid M. Naseem, Centre for Cardiovascular and Metabolic Research, Hull York Medical School, University of Hull, Cottingham Rd, Hull, United Kingdom; E-mail: khalid.naseem@hyms.ac.uk.