In this issue of Blood, Monnereau and colleagues pool 4 retrospective (case-control), observational epidemiologic studies to demonstrate an inverse association between UV radiation (UVR) exposure and risk of developing Hodgkin lymphoma (HL).1
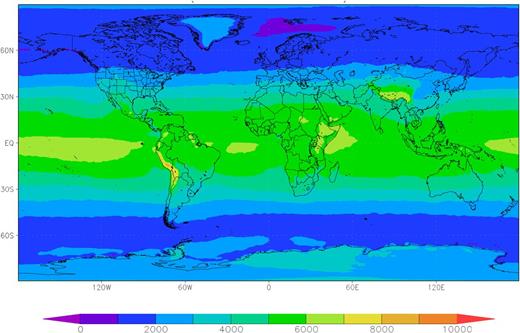
Global mean erythemal UVR daily dose (J/m2) during 2011-2012, based on data from the Ozone Monitoring Instrument on the National Aeronautics and Space Administration's Aura satellite as cited in Levelt et al.6 In addition to personal behaviors such as the amount of time spent outside and use of sun-protective clothing, ambient UVR is an important consideration in estimating UVR exposure histories because of its substantial variation by geographical location. Figure created by Dr Douglas C. Morton (NASA’s Goddard Space Flight Center, Greenbelt).
Global mean erythemal UVR daily dose (J/m2) during 2011-2012, based on data from the Ozone Monitoring Instrument on the National Aeronautics and Space Administration's Aura satellite as cited in Levelt et al.6 In addition to personal behaviors such as the amount of time spent outside and use of sun-protective clothing, ambient UVR is an important consideration in estimating UVR exposure histories because of its substantial variation by geographical location. Figure created by Dr Douglas C. Morton (NASA’s Goddard Space Flight Center, Greenbelt).
In recent years there has been considerable interest in the investigation of UVR and lymphoid malignancies. Most of the research has focused on non-HL, with initially conflicting data ultimately yielding to generally consistent inverse associations, although the biological mechanism underlying the relation is unclear. Fewer investigations have considered a potential association between UVR and HL, likely due in part to the relative rarity of the disease. However, in this issue of Blood, Monnereau et al1 advance the field by pooling data from 4 case-control studies of HL conducted in Europe, reporting that UVR is associated with reduced risk of HL, particularly Epstein-Barr virus (EBV)-positive HL (pooled odds ratio = 0.56, 95% confidence interval 0.35-0.91 for the highest vs lowest UVR exposure).
Pooling data across epidemiologic studies has key strengths and weaknesses that merit discussion. The main advantage lies in the large sample size, particularly for investigation of disease or patient subgroups. Additionally, pooled studies use individual-level data, which, unlike meta-analyses, allow exposure variables in each study to be redefined to a common scale. On the other hand, as Monnereau et al acknowledge,1 these harmonized variables may in fact obscure variability in exposure definitions arising from differences in the wording and structure of study questionnaires. More importantly, the detailed exposure characterization that may make an individual study so valuable is unlikely to be similar across studies, so the range of exposure variables that can be considered in a pooled analysis is often limited.
Monnereau et al leveraged the strengths of a pooled analysis to provide the first investigation of personal history of UVR exposure and HL according to disease subtype, taking into account HL histology as well as tumor EBV status.1 Interestingly, the data suggest that the inverse association may be stronger for EBV-positive than EBV-negative tumors. Although the finding requires confirmation, evidence for etiologic heterogeneity within HL has accumulated since the idea was first proposed nearly half a century ago2 and should be a priority for future investigation.
The measurement of UVR exposure is particularly complex, and the current report was somewhat hindered by the data available in the individual studies. The primary exposure metric was overall relative UVR exposure, calculated for each individual based on self-reported data in various formats, including time outdoors on working and nonworking days (2 studies), time outdoors doing routine leisure activities (2 studies), or frequency of sunbathing in the summertime (1 study). All 4 studies also had at least some data on sunlamp use.
Three main issues with the exposure assessment indicate that more research is needed to evaluate the potential association between UVR and HL. First, as a retrospective study, Monnereau et al relied on recollections made after HL diagnosis regarding time spent outdoors,1 which may have been distorted by the status of being ill. Although prospective studies would be small, they could provide useful confirmatory evidence. Second, Monnereau et al combined occupational and recreational exposures into a single metric,1 but 2 of the studies that contributed data to this pooled analysis reported a null or nonsignificantly positive association for occupational UVR and HL.3,4 Inconsistency in the findings between occupational and recreational exposures may harbor a clue to the disease association, because occupational exposures are frequently chronic, whereas recreational exposures are intermittent. Alternatively, the inconsistency may suggest a role for recall bias in reporting recreational exposures, because occupational exposures are likely to be more accurately reported. Finally, Monnereau et al1 did not take ambient UVR into account. Although personal behaviors contribute considerably to the variability in UVR exposure,5 the substantial variability in UVR by location (see figure6 ) modifies the impact of these behaviors. Indeed, ambient exposure has been used as an informative metric for UVR exposure in epidemiologic studies, with 2 recent such studies reporting conflicting results for HL.7,8
If the association between UVR and HL is causal, then the biological mechanism by which UVR contributes to lymphomagenesis is unclear. Previous research has focused on a potential role for vitamin D, which is generated by cutaneous sun exposure, has receptors expressed on most lymphocytes, and has been shown in vitro to impact the immune system in various ways.1,7 Despite excitement about this hypothesis, a large pooled analysis of 10 cohort studies showed no association for circulating 25-hydroxyvitamin D with non-HL,9 and the only study to examine circulating 25-hydroxyvitamin D and HL also was null,10 though these studies were limited by the use of a single measure of vitamin D. Alternative pathways, such as UVR induction of regulatory T cells, also are plausible but require further investigation.
Few modifiable risk factors for HL have been identified, and the scientific evidence is insufficient to conclude that either UVR or vitamin D is related to HL risk. However, the results of this pooled study should catalyze further research into UVR and HL. In addition to molecular studies, further epidemiologic research that can address key methodologic issues such as consideration of HL subtypes, recall bias, and integration of personal behaviors, occupational exposures, and ambient UVR has the greatest potential for shedding light on the etiology of HL.
Conflict-of-interest disclosure: The authors declare no competing financial interests.