Key Points
Risk-adapted therapy and broad use of HSCT resulted in a significant improvement in outcome.
AUTO- or ALLO-HSCT in high-risk patients resulted in a cumulative incidence of leukemia relapse superimposable to that of SR.
Abstract
We evaluated the outcome of 482 children with acute myeloid leukemia (AML) enrolled in the Associazione Italiana di Ematologia e Oncologia Pediatrica AML 2002/01 trial. Treatment was stratified according to risk group; hematopoietic stem cell transplantation (HSCT) was used in high-risk (HR) children. Patients with core binding factor leukemia achieving complete remission (CR) after the first induction course were considered standard risk (SR; 99 patients), whereas the others (n = 383) were assigned to the HR group. Allogeneic (ALLO) or autologous (AUTO) HSCT was employed, respectively, in 141 and 102 HR patients after consolidation therapy. CR, early death, and induction failure rates were 87%, 3%, and 10%, respectively. Relapse occurred in 24% of patients achieving CR. The 8-year overall survival (OS), event-free survival (EFS), and disease-free survival (DFS) were 68%, 55%, and 63%, respectively. OS, EFS, and DFS for SR and HR patients were 83%, 63%, and 66% and 64%, 53%, and 62%. DFS was 63% and 73% for HR patients given AUTO-HSCT and ALLO-HSCT, respectively. In multivariate analysis, risk group, white blood cell >100 × 109/L at diagnosis, and monosomal karyotype predicted poorer EFS. Risk-oriented treatment and broad use of HSCT result in a long-term EFS comparing favorably with previously published studies on childhood AML.
Introduction
The prognosis of childhood acute myeloid leukemia (AML) has significantly improved over the last 2 decades.1,2 In particular, the most recent and successful reports showed a probability of event-free survival (EFS) ranging between 40% and 60%.3-11 This improvement was largely due to (1) significant progress in stratification of patients, with a consequent risk-directed therapy; (2) optimization in induction and postremission treatment strategy, including the use of repeated courses of high-dose cytarabine (HD Ara-C); (3) better supportive therapy; and (4) broad use of allogeneic (ALLO) hematopoietic stem cell transplantation (HSCT) in high-risk (HR) patients.3 In this regard, in the past, randomized trials showed that ALLO-HSCT from an HLA-identical sibling is the postremission treatment able to offer the best chance of sustained remission.12-14 The efficacy of ALLO-HSCT from an HLA-matched sibling has been recently confirmed in the subsets of patients with intermediate/HR features.15 Despite these data, in the last years, and in view of an improved probability for relapsed patients to be rescued by salvage therapy, the use of ALLO-HSCT in children with AML in first complete remission (CR1) has been questioned by some groups.16,17 The role of autologous (AUTO) HSCT as postremission therapy for AML is even more controversial, and some studies have suggested an advantage over chemotherapy in terms of prevention of leukemia relapse,18 which, after the introduction of HD Ara-C in consolidation therapy, has not been confirmed12-14 or was offset by an increased risk of transplantation-related mortality (TRM).19
Since December 2002, children without Down syndrome and with newly diagnosed, de novo AML other than promyelocytic leukemia (APL) were treated at Italian centers affiliated with Associazione Italiana di Ematologia e Oncologia Pediatrica (AIEOP) according to the AML 2002/01 protocol, the latest of 5 consecutive studies on childhood AML (LAM-82, LAM-87, LAM-87M, LAM-92, and AML 2002/01) conducted in Italy since 1982.4 The primary objective chosen when the study was designed was that of obtaining a 5-year EFS >50%. In order to reach this goal, in view of the evidence available at that time on factors influencing outcome, we stratified children according to cytogenetic/molecular findings and response to the first course of induction therapy. In particular, assigned to the standard risk (SR) treatment were children with either AML1-ETO fusion transcript or anomalies of core binding factor β (CBF-β) and in morphologic CR after the first of 2 induction courses. In all patients, consolidation therapy included courses containing HD Ara-C. It was also decided that patients allocated to the HR group were broadly offered either AUTO- or ALLO-HSCT, depending on the availability of an HLA-identical sibling.
In this report, we describe the results obtained in children enrolled in the AML 2002/01 protocol.
Patients and methods
Eligibility
The entry criteria for the AIEOP AML 2002/01 study included (1) newly diagnosed de novo AML other than APL; (2) age ranging from 0 to 18 years; and (3) written informed consent from parents or legal guardians in accordance with the Declaration of Helsinki. Other eligibility criteria included serum bilirubin ≤3× upper limit of normal (ULN) for age, aspartate aminotransferase and alanine aminotransferase ≤5× ULN, and serum creatinine ≤2× ULN for age. Patients with a previous myelodysplastic phase or who had received previous treatment with either cytotoxic agents or steroids during the 2 weeks preceding diagnosis were excluded. The study was approved by the local ethics committees of each participating institution.
Diagnostic procedure
The initial diagnosis of AML with definition of subtype was established according to the French-American-British (FAB) and World Health Organization classification criteria. Bone marrow (BM) smears obtained at time of diagnosis were centrally reviewed at the Laboratory of Pediatric Hematology in Padua. All samples at diagnosis were analyzed for the presence of t(8;21), inv(16), t(16;16), t(15;17), and t(11q23)/mixed-lineage leukemia (MLL) and the related molecular transcripts, namely AML-ETO1, CBF-β abnormalities, promyelocytic leukemia-retinoic acid receptor α, and MLL rearrangements. Molecular analyses for internal tandem duplication of FLT-3 mutation (FLT3-ITD) and activating loop mutations of the same gene were also performed. The diagnosis of both FAB M0 and M7 subtypes was always confirmed by immunophenotype.
Morphologic CR status and diagnosis of relapse were centrally reviewed.
Definitions
CR was defined as <5% morphologically evident leukemic blasts in BM with normal hematopoiesis, no leukemia cells in peripheral blood or anywhere else, and signs of normal blood cell production (platelets >50 × 109/L without support, neutrophils > 1.0 × 109/L) after induction phase. Early death (ED) was defined as a fatal event occurring within the first 6 weeks from diagnosis.
Patients who did not achieve CR and who survived after the first course of treatment were divided in 2 groups: (1) partial responders (PR) were patients with percentage of BM leukemia blasts between 5% and 25% at the end of the first course of induction therapy; and (2) nonresponders (NR) were patients with percentage of BM leukemia blasts >25% at the end of the first course of induction therapy or patients with BM leukemia blasts >5% after the second induction course.
Central nervous system (CNS) involvement was defined as >5 leukocytes per μL of CSF and presence of leukemia cells on cytospin preparations or cranial nerve involvement.
Treatment design
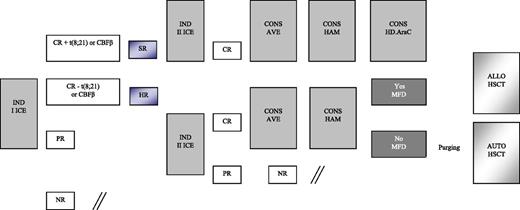
Details on treatment are reported in Figure 1 and Table 1. Patients enrolled in the AIEOP AML 2002/01 study were assigned to either the SR or HR group. As mentioned above, patients belonging to the former group had isolated anomalies of CBF-β and were in morphologic CR after the first induction course; the remaining children were assigned to the HR group. All patients, irrespective of the risk group, were given 2 courses of induction chemotherapy, including idarubicin, cytarabine, and etoposide (ICE; see Table 1 for details). BM evaluation to document response to the first induction course was on day +21. The second induction course for patients achieving CR was scheduled to start at time of hematologic recovery and preferably no later than 28 days after the beginning of the first course, in the absence of complications precluding cytotoxic treatment. PR and NR patients started either the second cycle of induction or salvage therapy, respectively, at time of evidence of leukemia persistence. Children in CR after the second induction course received 2 consolidation courses, including HD Ara-C, combined with etoposide in the first course (AVE; Table 1) and mitoxantrone in the second course (HAM; Table 1). At the end of this treatment, SR patients still in CR1 were given a further course of HD Ara-C (Table 1), while HR patients had the indication to receive either ALLO- or AUTO-HSCT, depending on the availability of an HLA-identical family donor. Among HR patients, those <1 year of age, with AML-M7, or not in CR at the end of first ICE, or with a complex karyotype were considered at particularly HR of recurrence and, thus, eligible to be transplanted from unrelated donors (UD). After 2006, all patients with FLT3-ITD in CR1 were also offered an UD allograft. NR patients were eligible for salvage therapy.
Schematic representation of the induction and consolidation courses employed for treating patients enrolled in the AIEOP AML 2002/01 trial. AVE, HD Ara-C and etoposide; CONS, consolidation; HAM, HD Ara-C and mitoxantrone; MFD, matched family donor.
Schematic representation of the induction and consolidation courses employed for treating patients enrolled in the AIEOP AML 2002/01 trial. AVE, HD Ara-C and etoposide; CONS, consolidation; HAM, HD Ara-C and mitoxantrone; MFD, matched family donor.
AIOP LAM 2002 chemotherapy schedule
| Phase . | Week . | Administration route . | Doses . | Days . |
|---|---|---|---|---|
| Remission induction (ICE ×2) | ||||
| Idarubicin | 1, 4 | IV | 10 mg/m2 | 1, 2, 3 |
| Etoposide | 1, 4 | IV | 100 mg/m2 | 1, 2, 3, 4, 5 |
| Cytarabine | 1, 4 | IV (24 h) | 200 mg/m2 | 1, 2, 3, 4, 5, 6, 7 |
| IT therapy | 1, 4 | |||
| Cytarabine | 1, 4 | IT | Depending on age* | 1 |
| Methotrexate (only for CNS disease) | 1, 4 | IT | Depending on age* | 1, 4, 7, 10, 14 of first cycle |
| Methylprednisolone (only for CNS disease) | 1, 4 | IT | Depending on age* | 1, 4, 7, 10, 14 of first cycle |
| Consolidation 1 (AVE) | ||||
| Etoposide | 8 | IV | 125 mg/m2 | 2, 3, 4, 5 |
| Cytarabine | 8 | IV | 3 g/m2 bid | 1, 2, 3 |
| IT therapy | ||||
| Cytarabine | 8 | IT | Depending on age* | 6 |
| Consolidation 2 (HAM) | ||||
| Cytarabine | 11 | IV | 3 g/m2 bid | 1, 2, 3 |
| Mitoxantrone | 11 | IV | 10 mg/m2 | 3, 4 |
| IT therapy | ||||
| Cytarabine | 11 | IT | Depending on age* | 6 |
| Consolidation 3 (HD Ara-C) (only for SR) | ||||
| Cytarabine | 14 | IV | 3 g/m2 bid | 1, 2, 3 |
| IT therapy | ||||
| Cytarabine | 14 | IT | Depending on age* | 6 |
| Phase . | Week . | Administration route . | Doses . | Days . |
|---|---|---|---|---|
| Remission induction (ICE ×2) | ||||
| Idarubicin | 1, 4 | IV | 10 mg/m2 | 1, 2, 3 |
| Etoposide | 1, 4 | IV | 100 mg/m2 | 1, 2, 3, 4, 5 |
| Cytarabine | 1, 4 | IV (24 h) | 200 mg/m2 | 1, 2, 3, 4, 5, 6, 7 |
| IT therapy | 1, 4 | |||
| Cytarabine | 1, 4 | IT | Depending on age* | 1 |
| Methotrexate (only for CNS disease) | 1, 4 | IT | Depending on age* | 1, 4, 7, 10, 14 of first cycle |
| Methylprednisolone (only for CNS disease) | 1, 4 | IT | Depending on age* | 1, 4, 7, 10, 14 of first cycle |
| Consolidation 1 (AVE) | ||||
| Etoposide | 8 | IV | 125 mg/m2 | 2, 3, 4, 5 |
| Cytarabine | 8 | IV | 3 g/m2 bid | 1, 2, 3 |
| IT therapy | ||||
| Cytarabine | 8 | IT | Depending on age* | 6 |
| Consolidation 2 (HAM) | ||||
| Cytarabine | 11 | IV | 3 g/m2 bid | 1, 2, 3 |
| Mitoxantrone | 11 | IV | 10 mg/m2 | 3, 4 |
| IT therapy | ||||
| Cytarabine | 11 | IT | Depending on age* | 6 |
| Consolidation 3 (HD Ara-C) (only for SR) | ||||
| Cytarabine | 14 | IV | 3 g/m2 bid | 1, 2, 3 |
| IT therapy | ||||
| Cytarabine | 14 | IT | Depending on age* | 6 |
CNS disease was considered as presence of >5 leukemia blasts in the cerebrospinal fluid, or nerve palsy or CNS involvement, at either nuclear magnetic resonance or at computed tomography scan.
AVE, high-dose cytarabine; bid, twice daily; HAM, HD Ara-C and mitoxantrone; IT, intrathecal; IV, intravenous.
Children <1 year received 20 mg cytarabine, while those aged between 1 and 2, or 2 and 3, or >3 years were given 30, 50, and 70 mg, respectively.
Conditioning regimen combined busulfan (16 mg/kg), cyclophosphamide (120 mg/kg), and melphalan (L-PAM; 140 mg/m2) for both AUTO- and ALLO-HSCT.20 Busulfan dosage was adjusted based on the pharmacokinetic study performed following the first administration in order to maintain a steady-state concentration between 600 and 900 ng/mL. Patients given AUTO-HSCT were recommended to have in vitro marrow purging with mafosfamide.21
Statistical analysis
The analysis used June 30, 2012, as the reference date.
Overall survival (OS) was calculated from date of diagnosis to time of death due to any cause or time of last contact. EFS was calculated from date of diagnosis to last follow-up or first event (failure to achieve CR, relapse, second malignancy, or death due to any cause, whichever occurred first). Patients who did not attain CR after 2 induction cycles were considered failures at time of remission evaluation. Disease-free survival (DFS) was calculated from the date of remission for both SR and HR patients reaching CR1 or, for the HR children given transplantation, from date of HSCT to last follow-up or first event (relapse, second malignancy, or death due to any cause, whichever occurred first).
Probabilities of OS, EFS, and DFS were estimated using the Kaplan-Meier method. Cumulative incidence (CI) of relapse and death in continuous CR (CCR) were constructed using the method of Kalbfleisch in order to adjust the analysis for competing risks. Death in remission was treated as a competing event to calculate CI of relapse, while relapse was considered the competing event for death in CCR. The significance of differences among the OS, EFS, and DFS curves was estimated by the log-rank test (Mantel-Cox), while Gray’s test was used to assess differences between CI of relapse and death in CCR. All variables having a P value < .05 in univariate analysis were included in a multivariate analysis on EFS using the Cox proportional regression model. Computations were performed using SAS (SAS Institute, Cary, NC).
Results
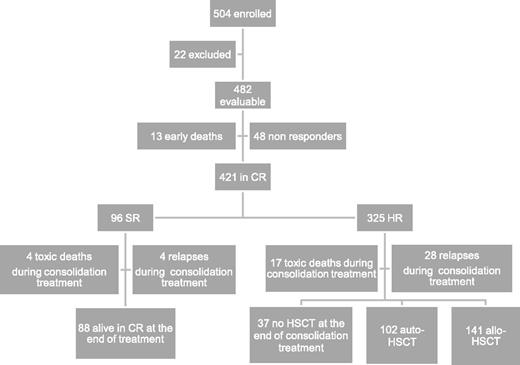
Between December 2002 and June 2011, 504 children with de novo AML other than APL were enrolled in the study; 482 of them are evaluable. Twenty-two were excluded due to death before initiation of therapy (n = 3), previous treatment with steroids (n = 7) or with cytotoxic drugs (n = 5), and previous diagnosis of myelodysplastic syndrome (n = 7). Children were diagnosed and treated at 29 centers affiliated with the AIEOP network; the number of patients aged <14 years treated per year was consistent with the expected number of new cases in Italy.22 Each center treated a number of patients ranging from 2 to 69 (median value: 9). Patient characteristics are shown in Table 2, while patient flowchart is depicted in Figure 2.
Patient characteristics of the AIEOP LAM 2002/01 protocol
| Characteristic . | n . | % . |
|---|---|---|
| Number of evaluable patients | 482 | 100 |
| Gender | ||
| Male | 262 | 52 |
| Female | 220 | 48 |
| Age | ||
| <1 y | 63 | 13 |
| 1-2 y | 52 | 10 |
| 2-10 y | 181 | 38 |
| >10 y | 186 | 39 |
| WBC count (×109/L) | ||
| <10 | 171 | 35 |
| 10-99 | 236 | 49 |
| >100 | 75 | 16 |
| CNS leukemia (yes) | 40 | 8 |
| FAB subtype | ||
| M0 | 34 | 7 |
| M1 | 88 | 18 |
| M2 | 91 | 19 |
| M4 | 40 | 8 |
| M4 Eo | 43 | 9 |
| M5 | 117 | 24 |
| M6 | 5 | 1 |
| M7 | 44 | 9 |
| Unclassifiable/not known | 20 | 4 |
| Cytogenetics | ||
| Patients with available cytogenetic data | 418 | |
| Favorable cytogenetics | 99 | 24 |
| t(8;21) | 72 | 17 |
| t(16;16) | 1 | 0 |
| inv 16 | 26 | 6 |
| Translocation t(9;11) | 19 | 5 |
| Other 11q23 abnormalities | 46 | 11 |
| Complex karyotype | 27 | 6 |
| Normal | 166 | 39 |
| Other abnormalities | 61 | 15 |
| FLT3 aberrations | ||
| Patients tested for FLT3 aberrations | 384 | |
| ITD | 42 | 11 |
| Activating loop mutations (D835/I836) | 10 | 3 |
| Wild-type | 332 | 86 |
| Other molecular aberrations | ||
| Patients tested | 244 | |
| NPM mutations | 14 | 6 |
| CEBPα mutations | 18 | 7 |
| Risk groups | ||
| SR | 99 | 20 |
| HR | 383 | 80 |
| Characteristic . | n . | % . |
|---|---|---|
| Number of evaluable patients | 482 | 100 |
| Gender | ||
| Male | 262 | 52 |
| Female | 220 | 48 |
| Age | ||
| <1 y | 63 | 13 |
| 1-2 y | 52 | 10 |
| 2-10 y | 181 | 38 |
| >10 y | 186 | 39 |
| WBC count (×109/L) | ||
| <10 | 171 | 35 |
| 10-99 | 236 | 49 |
| >100 | 75 | 16 |
| CNS leukemia (yes) | 40 | 8 |
| FAB subtype | ||
| M0 | 34 | 7 |
| M1 | 88 | 18 |
| M2 | 91 | 19 |
| M4 | 40 | 8 |
| M4 Eo | 43 | 9 |
| M5 | 117 | 24 |
| M6 | 5 | 1 |
| M7 | 44 | 9 |
| Unclassifiable/not known | 20 | 4 |
| Cytogenetics | ||
| Patients with available cytogenetic data | 418 | |
| Favorable cytogenetics | 99 | 24 |
| t(8;21) | 72 | 17 |
| t(16;16) | 1 | 0 |
| inv 16 | 26 | 6 |
| Translocation t(9;11) | 19 | 5 |
| Other 11q23 abnormalities | 46 | 11 |
| Complex karyotype | 27 | 6 |
| Normal | 166 | 39 |
| Other abnormalities | 61 | 15 |
| FLT3 aberrations | ||
| Patients tested for FLT3 aberrations | 384 | |
| ITD | 42 | 11 |
| Activating loop mutations (D835/I836) | 10 | 3 |
| Wild-type | 332 | 86 |
| Other molecular aberrations | ||
| Patients tested | 244 | |
| NPM mutations | 14 | 6 |
| CEBPα mutations | 18 | 7 |
| Risk groups | ||
| SR | 99 | 20 |
| HR | 383 | 80 |
CEBPα, CCAAT/enhancer binding protein; ITD, internal tandem duplication; NPM, nucleophosmin.
Overall, 87% of 482 patients achieved CR after induction, the early death and induction failure rates being 3% and 10%, respectively. In detail, 13 children died during induction courses and 48 were NR at the end of induction therapy (see also Figure 2). Among these 48 patients, 16 died due to either disease progression or treatment-related complications, while 32 (67%) subsequently reached CR after salvage therapy.
After the first course of induction therapy, 99 children were assigned to SR (20% of the whole population) as they had anomalies of CBF-β and were in morphological CR (Table 3). Notably, none of the children with CBF-β anomalies were resistant to the first induction course; thus, the CR rate of these children was 100%. Three SR patients died between the first and second ICE course, and thus the number of SR patients at the end of the cycles of induction therapy was 96 (23% of those being in CR after induction therapy). The remaining 325 children (77%) belonged to the HR group (see also Figure 2). The CR rate of children other than those with CBF-β anomalies was 85%. The median time elapsing between the beginning of the first and second course of induction therapy was 28 days (range 21-66 days).
Characteristics of SR and HR patients
| . | SR patients n (%) . | HR patients n (%) . | P value . |
|---|---|---|---|
| Number of evaluable patients | 99 (100) | 383 (100) | |
| Gender | |||
| Male | 59 (60) | 203 (53) | .6 |
| Female | 40 (40) | 180 (47) | .6 |
| Age | |||
| <1 y | 2 (2) | 61 (16) | .01 |
| 1-2 y | 7 (7) | 45 (12) | .1 |
| 2-10 y | 43 (43) | 138 (36) | .5 |
| >10 y | 47 (47) | 139 (36) | .3 |
| WBC count (×109/L) | |||
| <10 | 31 (31) | 140 (36) | .6 |
| 10-99 | 57 (58) | 179 (47) | .3 |
| >100 | 11 (11) | 64 (17) | .3 |
| CNS leukemia (yes) | 4 (4) | 36 (9) | .2 |
| FAB subtype | |||
| M0 | 0 (0) | 34 (9) | <.01 |
| M1 | 5 (5) | 83 (22) | .02 |
| M2 | 60 (61) | 31 (8) | <.01 |
| M4 | 10 (10) | 30 (8) | .8 |
| M4 Eo | 17 (17) | 26 (7) | .08 |
| M5 | 2 (2) | 115 (30) | <.01 |
| M6 | 0 (0) | 5 (1) | 1.0 |
| M7 | 0 (0) | 44 (11) | <.01 |
| Unclassifiable/not known | 5 (5) | 15 (4) | 1.0 |
| FLT3 aberrations | |||
| Patients tested for FLT3 aberrations | 73 | 311 | |
| ITD | 0 (0) | 42 (13) | <.01 |
| ALM (D835/I836) | 2 (3) | 8 (3) | 1.0 |
| Wild-type | 71 (96) | 261 (84) | .5 |
| Other molecular aberrations | |||
| Patients tested | 59 | 185 | |
| NPM mutations | 0 (0) | 14 (8) | <.01 |
| CEBPα mutations | 0 (0) | 18 (10) | <.01 |
| Outcome | |||
| Dead during induction | 3 (3) | 10 (3) | 1.0 |
| Dead in CR after induction | 4 (4) | 33 (7) | .5 |
| Relapsed | 24 (24) | 79 (21) | .7 |
| Salvaged after relapse | 17 (17) | 13 (3) | <.01 |
| Dead during/after salvage therapy | 3 (3) | 16 (4) | 1 |
| Dead after relapse | 4 (4) | 50 (13) | .04 |
| . | SR patients n (%) . | HR patients n (%) . | P value . |
|---|---|---|---|
| Number of evaluable patients | 99 (100) | 383 (100) | |
| Gender | |||
| Male | 59 (60) | 203 (53) | .6 |
| Female | 40 (40) | 180 (47) | .6 |
| Age | |||
| <1 y | 2 (2) | 61 (16) | .01 |
| 1-2 y | 7 (7) | 45 (12) | .1 |
| 2-10 y | 43 (43) | 138 (36) | .5 |
| >10 y | 47 (47) | 139 (36) | .3 |
| WBC count (×109/L) | |||
| <10 | 31 (31) | 140 (36) | .6 |
| 10-99 | 57 (58) | 179 (47) | .3 |
| >100 | 11 (11) | 64 (17) | .3 |
| CNS leukemia (yes) | 4 (4) | 36 (9) | .2 |
| FAB subtype | |||
| M0 | 0 (0) | 34 (9) | <.01 |
| M1 | 5 (5) | 83 (22) | .02 |
| M2 | 60 (61) | 31 (8) | <.01 |
| M4 | 10 (10) | 30 (8) | .8 |
| M4 Eo | 17 (17) | 26 (7) | .08 |
| M5 | 2 (2) | 115 (30) | <.01 |
| M6 | 0 (0) | 5 (1) | 1.0 |
| M7 | 0 (0) | 44 (11) | <.01 |
| Unclassifiable/not known | 5 (5) | 15 (4) | 1.0 |
| FLT3 aberrations | |||
| Patients tested for FLT3 aberrations | 73 | 311 | |
| ITD | 0 (0) | 42 (13) | <.01 |
| ALM (D835/I836) | 2 (3) | 8 (3) | 1.0 |
| Wild-type | 71 (96) | 261 (84) | .5 |
| Other molecular aberrations | |||
| Patients tested | 59 | 185 | |
| NPM mutations | 0 (0) | 14 (8) | <.01 |
| CEBPα mutations | 0 (0) | 18 (10) | <.01 |
| Outcome | |||
| Dead during induction | 3 (3) | 10 (3) | 1.0 |
| Dead in CR after induction | 4 (4) | 33 (7) | .5 |
| Relapsed | 24 (24) | 79 (21) | .7 |
| Salvaged after relapse | 17 (17) | 13 (3) | <.01 |
| Dead during/after salvage therapy | 3 (3) | 16 (4) | 1 |
| Dead after relapse | 4 (4) | 50 (13) | .04 |
ALM, activating loop mutations; CEBPα, CCAAT/enhancer binding protein; ITD, internal tandem duplication; NPM, nucleophosmin; Boldface P values denote statistically significant differences (<.05).
Thirty-seven patients died in CR after induction therapy; 33 and 4 out of these 37 children had been assigned to the HR and SR groups, respectively (P = .07). Of these 37 patients, 16 died after HSCT. The causes of death for the 21 patients who died in CR without or before having received HSCT were infections in 19 cases (6 of which were attributable to an invasive fungal infection) and cerebral hemorrhage in the remaining 2 children. The CI of death in CCR was 10% (standard error [SE] 1.7). Adolescents had a greater risk of death in CCR as compared with children aged <14 years (23% vs 6% in younger patients, P = .02). In detail, among the 14 adolescents who died in CR, 9 experienced fatal infections, 2 veno-occlusive disease after HSCT, 2 chronic graft-versus-host disease (GVHD), and 1 cerebral hemorrhage. Five adolescents died after HSCT.
Relapse occurred in 103 children (24%) who had achieved CR; 24 and 79 relapses occurred in SR and HR children, respectively. The median time between diagnosis and recurrence was 12.4 months (range 1.2-54.5 months). Four SR and 28 HR children relapsed while receiving consolidation therapy (see also Figure 2). Relapse involved BM only in 87 cases, BM and other extramedullary sites in 12 cases, and extramedullary sites in 4 cases only. The CI of relapse was 27% for both SR and HR patients (SE 4.7 and 2.8, respectively). Among the 24 SR patients who relapsed, 7 died of either disease progression (4 children) or toxicity during salvage treatment (3 children), while 17 patients are alive and disease-free in second CR. Of the 79 HR children experiencing leukemia recurrence, 66 died of either disease progression (50 children) or toxicity during salvage treatment (16 children), while 13 patients are alive and disease-free in second CR (see also Table 3).
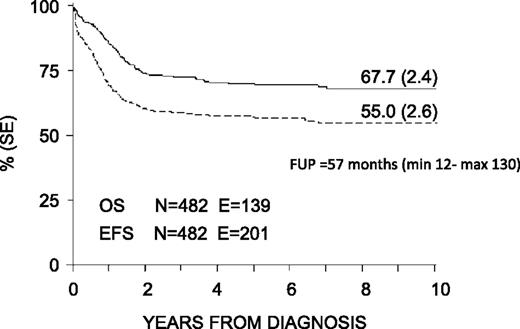
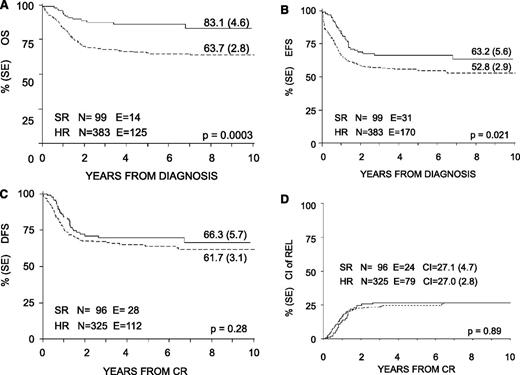
With a median follow-up of 57 months (range 12-130 months), the 8-year probability of OS, EFS, and DFS for the whole cohort was 68% (SE 2.4), 55% (SE 2.6), and 63% (SE 2.7), respectively (Figure 3). OS, EFS, and DFS were 83% (SE 4.6), 63% (SE 5.6), and 66% (SE 5.7) in the SR group; these values were 64% (SE 2.8), 53% (SE 2.9), and 62% (SE 3.1) in the HR group (Figure 4). Both the probability of OS and EFS were better for SR patients, with P values of .0003 and .02, respectively; by contrast, the probability of DFS did not differ between the 2 groups of risk. All patients with nucleophosmin and CCAAT/enhancer binding protein α were assigned to the HR group; their probability of EFS was 64% and 60%, respectively (see also Table 4).
Eight-year probability of OS and EFS for the whole cohort of 482 children enrolled in the AIEOP AML 2002/01 trial. FUP, follow-up.
Eight-year probability of OS and EFS for the whole cohort of 482 children enrolled in the AIEOP AML 2002/01 trial. FUP, follow-up.
Eight-year probability of OS, EFS, DFS, and CI of relapse for SR and HR children enrolled in the AIEOP AML 2002/01 trial. (A) OS. (B) EFS. (C) DFS. (D) CI of REL.
Eight-year probability of OS, EFS, DFS, and CI of relapse for SR and HR children enrolled in the AIEOP AML 2002/01 trial. (A) OS. (B) EFS. (C) DFS. (D) CI of REL.
Probability of EFS by patient subgroup
| Global population . | No. of patients . | EFS % (SE) . | P value . |
|---|---|---|---|
| Risk stratification | 482 | 55 (2.6) | |
| SR | 99 | 63 (5.6) | .021 |
| HR | 383 | 53 (2.9) | .021 |
| Age | |||
| <1 y | 63 | 58 (6.3) | .91 |
| 1-2 y | 52 | 58 (7.8) | .91 |
| 2-10 y | 181 | 55 (4.6) | .91 |
| >10 y | 186 | 57 (4.0) | .91 |
| WBC count (×109/L) | |||
| <10 | 171 | 60 (4.4) | .0001 |
| 10-99 | 236 | 57 (3.7) | .0001 |
| >100 | 75 | 37 (5.7) | .0001 |
| FAB types | |||
| M0 | 34 | 49 (8.9) | .12 |
| M1 | 88 | 44 (6.5) | .12 |
| M2 | 91 | 64 (5.8) | .12 |
| M4 | 83 | 64 (5.7) | .12 |
| M5 | 117 | 54 (5.6) | .12 |
| M6 | 5 | 100 (/) | .12 |
| M7 | 44 | 56 (7.7) | .12 |
| Unclassifiable/not known | 20 | 41(14.5) | .12 |
| Subgroups | |||
| FLT3 Pos overall | 52 | 47 (7,5) | |
| FLT3-ITD Pos | 42 | 47 (8.6) | |
| FLT3-ALM (D835/I836) Pos | 10 | 47 (6.7) | |
| FLT3-ITD Pos vs FLT3-ITD Neg | .22 | ||
| 11q23 abnormalities other than t(9;11) | 46 | 52 (10.1) | |
| 11q23 abnormalities other than t(9;11) vs other | .58 | ||
| t(9;11) | 19 | 61 (11.6) | |
| t(9;11) vs other | .78 | ||
| Monosomal karyotype | 10 | 20 (12.6) | |
| Monosomal karyotype vs other karyotype | .0002 | ||
| Complex karyotype | 27 | 40 (9.6) | |
| Complex karyotype vs other karyotype | .048 | ||
| NPM mutations Pos | 14 | 64 (15.2) | |
| NPM Pos vs NPM Neg | .24 | ||
| CEBPα mutations Pos | 18 | 60 (12.9) | |
| CEBPα Pos vs Neg | .33 |
| Global population . | No. of patients . | EFS % (SE) . | P value . |
|---|---|---|---|
| Risk stratification | 482 | 55 (2.6) | |
| SR | 99 | 63 (5.6) | .021 |
| HR | 383 | 53 (2.9) | .021 |
| Age | |||
| <1 y | 63 | 58 (6.3) | .91 |
| 1-2 y | 52 | 58 (7.8) | .91 |
| 2-10 y | 181 | 55 (4.6) | .91 |
| >10 y | 186 | 57 (4.0) | .91 |
| WBC count (×109/L) | |||
| <10 | 171 | 60 (4.4) | .0001 |
| 10-99 | 236 | 57 (3.7) | .0001 |
| >100 | 75 | 37 (5.7) | .0001 |
| FAB types | |||
| M0 | 34 | 49 (8.9) | .12 |
| M1 | 88 | 44 (6.5) | .12 |
| M2 | 91 | 64 (5.8) | .12 |
| M4 | 83 | 64 (5.7) | .12 |
| M5 | 117 | 54 (5.6) | .12 |
| M6 | 5 | 100 (/) | .12 |
| M7 | 44 | 56 (7.7) | .12 |
| Unclassifiable/not known | 20 | 41(14.5) | .12 |
| Subgroups | |||
| FLT3 Pos overall | 52 | 47 (7,5) | |
| FLT3-ITD Pos | 42 | 47 (8.6) | |
| FLT3-ALM (D835/I836) Pos | 10 | 47 (6.7) | |
| FLT3-ITD Pos vs FLT3-ITD Neg | .22 | ||
| 11q23 abnormalities other than t(9;11) | 46 | 52 (10.1) | |
| 11q23 abnormalities other than t(9;11) vs other | .58 | ||
| t(9;11) | 19 | 61 (11.6) | |
| t(9;11) vs other | .78 | ||
| Monosomal karyotype | 10 | 20 (12.6) | |
| Monosomal karyotype vs other karyotype | .0002 | ||
| Complex karyotype | 27 | 40 (9.6) | |
| Complex karyotype vs other karyotype | .048 | ||
| NPM mutations Pos | 14 | 64 (15.2) | |
| NPM Pos vs NPM Neg | .24 | ||
| CEBPα mutations Pos | 18 | 60 (12.9) | |
| CEBPα Pos vs Neg | .33 |
ALM, activating loop mutations; CEBPα, CCAAT/enhancer binding protein; ITD, internal tandem duplication; Neg, negative; NPM, nucleophosmin; Pos, positive; Boldface P values denote statistically significant differences (<.05).
Notably, the 8-year OS of the 48 NR patients was 30% (SE 7.6). Of them, 27 were given an allograft, either in CR or with persistent disease, from an HLA-identical sibling or an alternative donor.
Considering variables potentially influencing outcome (see Table 4 for details), we found that patients with white blood cell (WBC) count at diagnosis >100 × 109/L had a worse outcome as compared with the others, the EFS being 37% (SE 5.7) and 58% (SE 2.8), respectively (P < .05). The 8-year EFS of the 27 patients with complex karyotype (defined as ≥3 abnormalities, either numerical or structural) was 40% (SE 9.6) as compared with 56% (SE 2.6) in the other patients (P < .05). The EFS of patients carrying (n = 10) or not (n = 472) a monosomal karyotype, defined as the presence of isolated −7 and −5, was 20% (SE 12.6) and 53% (SE 2.9), respectively (P = .0002). Noteworthy, FLT3-ITD did not influence the probability of EFS (see also Table 3). In multivariate analysis for EFS, risk group (either SR or HR), WBC count at diagnosis >100 × 109/L, and monosomal karyotype predicted poor patient outcome (with P values of .001, .001, and .049 and hazard ratios of 1.95, 3.37, and 1.52, respectively).
As far as the outcome of the 243 patients given transplantation in CR1 is concerned, the 8-year DFS, calculated from the date of HSCT, for the 102 patients given AUTO-HSCT was 63% (SE 4.9); it was 73% (SE 4.0) for the 141 patients given ALLO-HSCT (P value not significant). Among this latter group, DFS was 71% (SE 6.3) and 79% (SE 6.2) for the 66 and 75 patients transplanted from a relative or an UD, respectively. Sixteen patients died after HSCT; causes of death were acute GVHD (n = 3), chronic GVHD (n = 4), veno-occlusive disease (n = 4), and infection (n = 5). The 8-year CI of TRM after either AUTO- or ALLO-HSCT from an HLA-compatible sibling or an UD was comparable (7% in both cohorts). The 8-year CI of relapse after AUTO- and ALLO-HSCT was 28% (SE 4.5) and 17% (SE 3.3), respectively (P = .043).
Thirty-seven HR patients (11% of the whole HR population) received neither AUTO- nor ALLO-HSCT at the end of consolidation courses (Figure 2) due to parent refusal (21 patients), physician decision (10 patients), or complications occurring during chemotherapy treatment and precluding transplantation (7 patients). These 37 HR patients did not differ from the other HR children and their OS and DFS probability, calculated from the end of consolidation chemotherapy, was 47% (SE 7.1) and 43% (SE 7.3), respectively. In comparison with HR patients given ALLO- and AUTO-HSCT, after adjusting for waiting time to transplantation (39 days, range 31-58 days), both DFS and OS of the 37 nontransplanted HR patients were lower (P = .033 and P = .041, respectively).
Discussion
The 8-year OS and EFS probabilities of 68% and 55%, respectively, achieved in this multicenter study of risk-adapted therapy, based on genetic features and response to induction therapy, are better than those reported in previous AIEOP studies.4 This improvement can be attributed to a lower risk of both treatment-related death and disease recurrence. In particular, the ED rate (3%) of the AML 2002/01 protocol compares favorably with those reported in the LAM-92, LAM-87M, LAM-87, and LAM-82 studies (6%, 14%, 5%, and 9%, respectively).4 Our ED rate is similar to that reported by the BFM5 and MRC groups6,23 but worse than those reported by a monoinstitutional study at St. Jude7 and by the NOPHO24 and Japanese pediatric groups.8 Also, the incidence of death in CCR of our study (10%) was comparable to that recently reported by Rubnitz et al7 (9%). We found that both HR patients and adolescents were exposed to a greater risk of experiencing fatal toxicities. This observation suggests that special attention ought to be paid to the supportive therapy in these categories of children. Previously published studies showed that, also in acute lymphoblastic leukemia, adolescents are more fragile than children.25-27 The reasons behind the observation that adolescents have an increased risk for death in CCR are unclear, but they may reflect age-dependent differences in immunologic response to infections and tissue toxicity following chemotherapy.
The OS and EFS of our cohort of patients are comparable or better than those reported by several other international cooperative groups.5,6,24,28,29 Two recent reports have shown better results, since the Japanese AML99 study reported a 5-year EFS and OS of 61% and 75%,8 respectively, and the AML02 single-center study from St. Jude reported an EFS and OS at 3 years of 63% and 71%, respectively.7 However, it is noteworthy that the number of patients enrolled in the AIEOP AML 2002/01 multicenter study is, together with that reported by Gibson and coworkers,23 the highest of the published protocols on childhood AML and that we report results at 8 years, thus with a follow-up longer than that of St. Jude and Japanese studies.7,8 Moreover, in our trial, neither patients with APL (enrolled in the BFM trial)5 nor those with Down syndrome (enrolled in the MRC trial) were included,6,30 and the proportion of patients with CBF-β abnormalities is lower than that of the Japanese study (20% vs 37%, respectively).8
The risk stratification based on genetic features and response to induction treatment, chosen in 2002 when the study was designed, has confirmed its prognostic value also in our cohort. Indeed, HR patients had both an OS and EFS probability worse than those of SR patients. However, the difference was less pronounced for EFS than for OS, since a higher proportion of SR patients than HR ones can be rescued by second-line therapies (Table 3); this finding is in line with a recent study reporting on the outcome of children with relapsed AML.31 The outcome of our SR patients was inferior to that reported by other groups.3,6-8,10,32 There is no obvious explanation for this result. Considering compliance with dose intensity as a possible reason for the outcome of our SR patients being worse than expected, the median treatment duration for SR patients was 170 days (range 135-210 days); the estimated median duration of treatment of SR patients was 140 days. This delay could have partly contributed to the unsatisfactory outcome of SR children. The new AIEOP study will address the question of whether the use of flow-cytometry–based minimal residual disease detection, which is certainly much more sensitive than morphologic evaluation for monitoring early leukemia clearance7 and/or early/repeated courses of mitoxantrone,32 can improve the EFS of SR children.
The worse outcome observed in patients with monosomal karyotype has been reported in adult patients,33 but it is a relatively novel observation for childhood AML, since only the negative impact of monosomy 7 has been previously described.10,11,34 In the study by Breems et al,33 out of 184 adults with monosomal karyotype, only 5 patients survived; similarly, in our study, only 2 out of 10 patients are alive and disease-free. Taken together, these results indicate that patients with monosomal karyotype should be considered at HR of treatment failure and should be offered the most effective treatments to prevent disease recurrence.
The outcome of children with WBC count at diagnosis >100 × 109/L was significantly worse than that of patients with lower counts, irrespective of their allocation to either the SR or the HR group (data not shown). This finding is in line with previously published reports,35 although more recent studies have documented that repeated courses of intensive chemotherapy may abrogate the impact on outcome of this variable.3,7
FLT3-ITD has been reported to be an adverse prognostic factor in both adults and children with AML.36-38 The observation that our 42 patients with FLT3-ITD, 20 of them given ALLO-HSCT, had an outcome not statistically different from that of HR patients without this molecular abnormality can be explained considering that these children, after 2006, were offered, if in CR and with an available HLA-matched donor, ALLO-HSCT, a treatment associated with an immune-mediated graft-versus-leukemia effect. Indeed, the DFS of our children given ALLO-HSCT was 75% +/− 15%. Previously published studies have demonstrated that ALLO-HSCT can supersede the negative prognostic impact of FLT3-ITD in AML.39,40
The broad use of either AUTO- or ALLO-HSCT in HR patients resulted into a CI of leukemia recurrence superimposable to that of SR children. This favorable effect of transplantation on the risk of recurrence was not offset by an unacceptable CI of TRM. In this regard, it is noteworthy that the outcome of ALLO-HSCT from UD was similar to that of patients given HLA-compatible sibling transplantation. This finding is backed by recent reports in patients with acute lymphoblastic leukemia41,42 or nonmalignant disorders43 and can be interpreted in view of optimization of HLA-typing techniques and strategies for preventing or treating GVHD. Children were given ALLO-HSCT in few experienced centers, while a larger number of centers performed AUTO-HSCT; this conceivably may have contributed to the comparable TRM observed in AUTO- and ALLO-HSCT recipients. Support to the role played by HSCT in preventing leukemia recurrence in our HR patients is also given by the worse OS and DFS calculated by the end of chemotherapy treatment observed in the 37 HR children who were not transplanted at the end of consolidation therapy.
The DFS observed in our HR children given ALLO-HSCT is comparable or better than that reported in other studies, which, however, sometimes also included SR children.8,16,23,44,45 In view of the remarkable chance of salvage recently reported in patients with AML who experience relapse,31,46 transplantation could have been spared in at least a proportion of our HR patients without jeopardizing the probability of OS. This consideration has particular value in view of the possible occurrence of late complications related to transplantation, namely loss of fertility, endocrine disturbances, growth impairment, and extensive chronic GVHD.47 The CI of this immune-mediated complication in our cohort was 9% (SE 2.5).
In conclusion, in a large cohort of children with a long follow-up, we show that risk-adapted therapy and broad use of HSCT resulted in a significant improvement in the outcome as compared with previous AIEOP protocols.4 The next AIEOP study will stratify patients into 3 risk groups, namely SR, HR, and very HR (VHR) children. The former will include children with CBF-β anomalies as well as cytogenetically normal, FLT3-ITD negative and nucleophosmin mutated children with early MRD clearance, measured through flow cytometry.7 In the VHR group, we will allocate children with (1) poor MRD clearance7 ; (2) WBC count at diagnosis >100 × 109/L in the absence of molecular lesions typical of SR; (3) AML FAB-M7 without t(1;22); (4) complex or monosomal karyotype; (5) FLT3-ITD; and (6) recently detected poor-prognosis molecular lesions.48,49 The remaining children will be assigned to the HR group. SR patients will be offered chemotherapy-only treatment, while HR children with an HLA-identical sibling available and all VHR children will be offered an allograft. We will explore whether this refined stratification permits us to further improve patient outcome and whether EFS of HR patients without an HLA-identical sibling can be reproduced by substituting AUTO-HSCT with chemotherapy courses including drugs such as fludarabine and amsacrine.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Barbara Buldini, Dr Laura Sainati, Dr Anna Leszl, and Dr Gianni Cazzaniga for their valuable help in the conception and design of the study and for fruitful discussions during trial conduction.
This work was supported in part by a grant from AIRC (Associazione Italiana Ricerca sul Cancro, Special Grant “5x1000”) (F.L.) and by a grant from Italian Ministry of University and Scientific Research (PRIN-2009) (S.R.) (PRIN-2010) (F.L.).
Authorship
Contribution: A.P. designed the study, treated patients, analyzed the data, and wrote the manuscript; R.M. collected and analyzed the data, treated patients, and wrote the manuscript; C.R., M.C.P., F.C., F.F., M.L., L.L.N., G.M., C.M., N.S., A.M.T., M.Z., and A.B. contributed to the design of the study, treated patients, and collected the data; M.P. performed polymerase chain reaction analyses for characterization of molecular lesions; S.R. analyzed the data and wrote the manuscript; R.R. checked and analyzed the data; G.B. contributed to the design of the study and was responsible for confirmation of diagnosis, flow cytometry analysis, and polymerase chain reaction analyses for characterization of molecular lesions; and F.L. designed the study, treated patients, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Franco Locatelli, Department of Pediatric Hematology/Oncology, IRCCS Bambino Gesù Children’s Hospital, Piazza Sant’Onofrio, 4, 00165 Rome, Italy; e-mail: franco.locatelli@opbg.net.