Key Points
t-CMML is associated with higher-risk cytogenetics and manifests poor prognosis.
t-CMML should be recognized as one of the therapy-related myeloid neoplasms.
Abstract
We sought to describe the clinical features and outcomes of therapy-related chronic myelomonocytic leukemia (t-CMML) and compare with those of de novo CMML. We identified 358 CMML patients, of whom 39 (11%) had t-CMML. Although the groups had similar demographic, hematologic, and molecular alteration profiles, the proportion of patients with intermediate or high CMML-specific cytogenetic risk in the t-CMML was significantly higher than that in the de novo CMML (P = .011). The median latency to develop t-CMML was 6 years. The median overall and leukemia-free survival duration of the t-CMML were shorter than those of the de novo CMML; however, t-CMML itself was not prognostic after adjusting for the effects of other covariates including cytogenetics. These results suggest that compared with de novo CMML, t-CMML is associated with more high-risk cytogenetics that manifest as poor outcomes. We propose that t-CMML be recognized as one of the therapy-related myeloid neoplasms.
Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and the American Society of Hematology.
Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 70% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 2920.
Disclosures
The authors, Associate Editor Richard A. Van Etten, and CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, declare no competing financial interests.
Learning objectives
Describe clinical features of therapy-related chronic myelomonocytic leukemia (t-CMML) and de novo CMML, based on a case series.
Compare cytogenetic risk in t-CMML with that in de novo CMML.
Compare prognosis in t-CMML with that in de novo CMML.
Release date: October 17, 2013; Expiration date: October 17, 2014
Introduction
Therapy-related myeloid malignancies are a distinct group of myeloid neoplasms that are associated with exposure to certain cytotoxic chemotherapies or ionizing radiation. Currently, the World Health Organization (WHO) defines 2 types of therapy-related myeloid neoplasms: (1) alkylating agent–related or radiation-related acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS); and (2) topoisomerase II inhibitor–related AML.1 In general, these therapy-related diseases are associated with high-risk cytogenetic abnormalities and respond poorly to conventional therapies.
Chronic myelomonocytic leukemia (CMML) is characterized by persistent peripheral blood monocytosis (>1.0 × 109/µL), a variable bone marrow blast of <20%, and dysplastic hematopoiesis.2 On rare occasions, CMML is diagnosed in patients who have been exposed to cytotoxic chemotherapies or ionizing radiation, hence considered as therapy-related CMML (t-CMML). Although several case reports of t-CMML have been identified,3-7 no systematic analysis of the disease’s clinical characteristics and prognosis have been conducted owing to its extreme rarity.
The present study aims to describe the clinical features and outcomes of t-CMML patients and compare these characteristics with those of de novo CMML patients.
Study design
We identified 358 CMML patients who were referred to MD Anderson Cancer Center (MDACC) between January 2003 and July 2012. The median time from outside diagnosis to MDACC referral was 1.5 months (range: 0-80). Of those patients, 39 (11%) had prior exposure to chemotherapy and/or radiation therapy and were defined as t-CMML. Clinical data of the studied patients were obtained at the time of referral to MDACC. Therapies that were given to the patients were categorized into 4 groups: (1) best supportive care or cytoreductive therapy using hydroxyurea or oral etoposide (BSC/CR); (2) hypomethylating agents (HMA) such as 5-azacitidine–based or decitabine-based regimens; (3) AML-like induction therapy; and (4) other therapies including immunomodulatory drugs (thalidomide or lenalidomide) or tyrosine kinase inhibitors (iMiDs/TKIs). Cytogenetics and mutational analyses of the FLT3, KRAS, NRAS, and JAK2 genes were performed as described previously.8-10 The study protocol followed the guidelines of the Declaration of Helsinki and was approved by the Institutional Review Board at MDACC.
Statistical methods and term definitions are described in the supplemental Methods; see the Blood Web site).
Results and discussion
The demographics and clinical characteristics of 319 de novo CMML and 39 t-CMML patients are given in Table 1. Cytogenetics was categorized by CMML-specific cytogenetic risk classification.11,12 The proportion of patients with intermediate- or high-risk cytogenetic abnormalities in the t-CMML group (50%) was significantly higher than that in the de novo CMML group (26%; P = .011). We used the MDAPS and CPSS to evaluate prognostic risk in the groups.11,13 Among the evaluable patients, both scoring systems were verified to define distinct subgroups among the de novo CMML patients (supplemental Figures 1A, 2A, and 3A), and they appeared to identify high-risk patients in the t-CMML group despite the small cohort size (supplemental Figures 1B, 2B, and 3B). Although the distributions of MDAPS-defined risk groups did not differ significantly, the proportion of patients with CPSS-defined low risk in the t-CMML group (3%) was significantly smaller than that in the de novo CMML group (25%; P = .024) (Table 1). This discrepancy in risk distribution likely occurred because the CPSS incorporates cytogenetics in its score calculation, whereas the MDAPS is based on hematologic parameters only. De novo and t-CMML patients received similar types of therapies (P = .537), and the proportion of patients who underwent SCT was not statistically different between the 2 groups (P = .077) (Table 1).
Clinical and demographic characteristics of 319 patients with de novo CMML and 39 patients with t-CMML
| Variable . | De novo CMML (N = 319) . | t-CMML (N = 39) . | P . |
|---|---|---|---|
| Median age, y (range) | 68 (23-89) | 69 (30-80) | .995 |
| Female | 101 (32) | 12 (31) | .910 |
| WHO classification | |||
| CMML-1 | 221 (69) | 18 (46) | .080 |
| CMML-2 | 95 (30) | 15 (38) | |
| Unknown | 3 (1) | 6 (15) | |
| Median WBC, ×103/µL (range) | 14.6 (1.1-223) | 11.3 (2.4-113.6) | .209 |
| Median HGB, g/dL (range) | 10.5 (5.1-17.1) | 10.8 (8.4-15.3) | .903 |
| Median PLT, ×103/µL (range) | 94.0 (5.0-809) | 90 (20-545) | .797 |
| Median AMC, ×103/µL (range) | 2.91 (0.02-101.76) | 2.81 (0.25-28.99) | .414 |
| Median ALC, ×103/µL (range) | 2.0 (0.08-13.5) | 1.67 (0.36-7.13) | .196 |
| IMCs | 215 (67) | 26 (68) | .899 |
| Median BM blast percentage (range) | 6 (0-19) | 7 (1-18) | .443 |
| Median LDH, IU/L (range) | 640 (191-7380) | 598 (327-3518) | .435 |
| Median β2MG, mg/dL (range) | 3.9 (1.2-21.4) | 3.7 (1.3-56.8) | .372 |
| Median lysozyme, µg/mL (range) | 20 (2.1-204.0) | 20.0 (10.6-34.5) | .918 |
| Cytogenetic abnormalities | |||
| Diploid | 209 (66) | 17 (44) | |
| -Y sole | 14 (4) | 0 (0) | |
| -Y plus 1 abn | 1 (0.3) | 0 (0) | |
| 3q sole | 2 (0.6) | 0 (0) | |
| del 5q/-5 sole | 1 (0.3) | 0 (0) | |
| del 5q/-5 plus 1 abn | 0 (0) | 0 (0) | |
| del 7q/-7 sole | 8 (3) | 1 (3) | |
| del 7q/-7 plus 1 abn | 9 (3) | 2 (5) | |
| +8 sole | 11 (3) | 0 (0) | |
| +8 plus 1 abn | 3 (0.9) | 0 (0) | |
| 9q sole | 1 (0.3) | 2 (5) | |
| 11q23 sole | 2 (0.6) | 0 (0) | |
| Del 12p sole | 2 (0.6) | 0 (0) | |
| Del 13q sole | 2 (0.6) | 2 (5) | |
| Del 20q sole | 6 (2) | 5 (13) | |
| del 20q plus 1 abn | 2 (0.6) | 0 (0) | |
| +21 sole | 4 (1) | 0 (0) | |
| +21 plus 1 abn | 1 (0.3) | 0 (0) | |
| Complex | 12 (4) | 5 (13) | |
| Other nonrecurrent abnormalities | 13 (4) | 0 (0) | |
| Unknown | 16 (5) | 5 (13) | |
| Cytogenetics risk category* | .011 | ||
| Low | 224 (74) | 17 (50) | |
| Intermediate | 36 (12) | 9 (26) | |
| High | 43 (14) | 8 (24) | |
| Molecular analysis, N/tested (%) | |||
| NRAS G12D | 46/251 (18) | 3/29 (10) | .251† |
| KRAS G12D | 15/251 (6) | 1/29 (3) | |
| FLT3-ITD | 10/268 (4) | 0/29 (0) | .606 |
| FLT3-D835 | 3/267 (1) | 0/29 (0) | .999 |
| JAK2 V617F | 18/149 (12) | 1/12 (8) | .999 |
| MDAPS-defined risk | .752 | ||
| Low | 105 (33) | 14 (37) | |
| Intermediate-1 | 99 (31) | 14 (37) | |
| Intermediate-2 | 65 (20) | 6 (16) | |
| High | 50 (16) | 4 (11) | |
| CPSS-defined risk | .010 | ||
| Low | 70 (25) | 1 (3) | |
| Intermediate-1 | 80 (29) | 13 (45) | |
| Intermediate-2 | 102 (37) | 10 (35) | |
| High | 25 (9) | 5 (17) | |
| Therapy‡ | .537 | ||
| BSC/CR | 112 (35) | 14 (36) | |
| HMA | 157 (49) | 16 (41) | |
| AML-like induction therapy | 15 (5) | 3 (8) | |
| iMiDs/TKIs | 35 (11) | 6 (15) | |
| SCT | 17 (5) | 5 (13) | .077 |
| Primary cancer types | |||
| Breast | NA | 5 (13) | |
| Prostate | NA | 12 (31) | |
| Hodgkin lymphoma | NA | 1 (3) | |
| Non-Hodgkin lymphoma | NA | 12 (31) | |
| Multiple myeloma | NA | 1 (3) | |
| Ovarian | NA | 2 (5) | |
| Head and neck/lung | NA | 3 (8) | |
| Gastrointestinal | NA | 1 (3) | |
| Unknown | NA | 2 (5) | |
| Exposure | |||
| Radiation only | NA | 15 (38) | |
| Chemotherapy or combined modality | NA | 24 (62) | |
| Exposure to alkylating agents | NA | 17 (44) | |
| Exposure to topoisomerase II inhibitor | NA | 4 (10) | |
| Autologous SCT | NA | 7 (18) | |
| Median latency, y (range)§ | NA | 6 (1-32) |
| Variable . | De novo CMML (N = 319) . | t-CMML (N = 39) . | P . |
|---|---|---|---|
| Median age, y (range) | 68 (23-89) | 69 (30-80) | .995 |
| Female | 101 (32) | 12 (31) | .910 |
| WHO classification | |||
| CMML-1 | 221 (69) | 18 (46) | .080 |
| CMML-2 | 95 (30) | 15 (38) | |
| Unknown | 3 (1) | 6 (15) | |
| Median WBC, ×103/µL (range) | 14.6 (1.1-223) | 11.3 (2.4-113.6) | .209 |
| Median HGB, g/dL (range) | 10.5 (5.1-17.1) | 10.8 (8.4-15.3) | .903 |
| Median PLT, ×103/µL (range) | 94.0 (5.0-809) | 90 (20-545) | .797 |
| Median AMC, ×103/µL (range) | 2.91 (0.02-101.76) | 2.81 (0.25-28.99) | .414 |
| Median ALC, ×103/µL (range) | 2.0 (0.08-13.5) | 1.67 (0.36-7.13) | .196 |
| IMCs | 215 (67) | 26 (68) | .899 |
| Median BM blast percentage (range) | 6 (0-19) | 7 (1-18) | .443 |
| Median LDH, IU/L (range) | 640 (191-7380) | 598 (327-3518) | .435 |
| Median β2MG, mg/dL (range) | 3.9 (1.2-21.4) | 3.7 (1.3-56.8) | .372 |
| Median lysozyme, µg/mL (range) | 20 (2.1-204.0) | 20.0 (10.6-34.5) | .918 |
| Cytogenetic abnormalities | |||
| Diploid | 209 (66) | 17 (44) | |
| -Y sole | 14 (4) | 0 (0) | |
| -Y plus 1 abn | 1 (0.3) | 0 (0) | |
| 3q sole | 2 (0.6) | 0 (0) | |
| del 5q/-5 sole | 1 (0.3) | 0 (0) | |
| del 5q/-5 plus 1 abn | 0 (0) | 0 (0) | |
| del 7q/-7 sole | 8 (3) | 1 (3) | |
| del 7q/-7 plus 1 abn | 9 (3) | 2 (5) | |
| +8 sole | 11 (3) | 0 (0) | |
| +8 plus 1 abn | 3 (0.9) | 0 (0) | |
| 9q sole | 1 (0.3) | 2 (5) | |
| 11q23 sole | 2 (0.6) | 0 (0) | |
| Del 12p sole | 2 (0.6) | 0 (0) | |
| Del 13q sole | 2 (0.6) | 2 (5) | |
| Del 20q sole | 6 (2) | 5 (13) | |
| del 20q plus 1 abn | 2 (0.6) | 0 (0) | |
| +21 sole | 4 (1) | 0 (0) | |
| +21 plus 1 abn | 1 (0.3) | 0 (0) | |
| Complex | 12 (4) | 5 (13) | |
| Other nonrecurrent abnormalities | 13 (4) | 0 (0) | |
| Unknown | 16 (5) | 5 (13) | |
| Cytogenetics risk category* | .011 | ||
| Low | 224 (74) | 17 (50) | |
| Intermediate | 36 (12) | 9 (26) | |
| High | 43 (14) | 8 (24) | |
| Molecular analysis, N/tested (%) | |||
| NRAS G12D | 46/251 (18) | 3/29 (10) | .251† |
| KRAS G12D | 15/251 (6) | 1/29 (3) | |
| FLT3-ITD | 10/268 (4) | 0/29 (0) | .606 |
| FLT3-D835 | 3/267 (1) | 0/29 (0) | .999 |
| JAK2 V617F | 18/149 (12) | 1/12 (8) | .999 |
| MDAPS-defined risk | .752 | ||
| Low | 105 (33) | 14 (37) | |
| Intermediate-1 | 99 (31) | 14 (37) | |
| Intermediate-2 | 65 (20) | 6 (16) | |
| High | 50 (16) | 4 (11) | |
| CPSS-defined risk | .010 | ||
| Low | 70 (25) | 1 (3) | |
| Intermediate-1 | 80 (29) | 13 (45) | |
| Intermediate-2 | 102 (37) | 10 (35) | |
| High | 25 (9) | 5 (17) | |
| Therapy‡ | .537 | ||
| BSC/CR | 112 (35) | 14 (36) | |
| HMA | 157 (49) | 16 (41) | |
| AML-like induction therapy | 15 (5) | 3 (8) | |
| iMiDs/TKIs | 35 (11) | 6 (15) | |
| SCT | 17 (5) | 5 (13) | .077 |
| Primary cancer types | |||
| Breast | NA | 5 (13) | |
| Prostate | NA | 12 (31) | |
| Hodgkin lymphoma | NA | 1 (3) | |
| Non-Hodgkin lymphoma | NA | 12 (31) | |
| Multiple myeloma | NA | 1 (3) | |
| Ovarian | NA | 2 (5) | |
| Head and neck/lung | NA | 3 (8) | |
| Gastrointestinal | NA | 1 (3) | |
| Unknown | NA | 2 (5) | |
| Exposure | |||
| Radiation only | NA | 15 (38) | |
| Chemotherapy or combined modality | NA | 24 (62) | |
| Exposure to alkylating agents | NA | 17 (44) | |
| Exposure to topoisomerase II inhibitor | NA | 4 (10) | |
| Autologous SCT | NA | 7 (18) | |
| Median latency, y (range)§ | NA | 6 (1-32) |
All data are no. of patients (%) unless otherwise specified.
ALC, absolute lymphocyte count; AMC, absolute monocyte count; β2MG, β2 microglobulin; BM, bone marrow; CPSS, CMML-specific prognostic scoring system; HGB, hemoglobin; IMCs, presence of immature myeloid cells in the peripheral blood; ITD, internal tandem duplication; LDH, lactate dehydrogenase; MDAPS, MD Anderson Prognostic Score; NA, not applicable; PLT, platelet count; SCT, stem cell transplant; WBC, white blood cell count.
Cytogenetic risk category was defined by CMML-specific cytogenetic risk category by Such et al.12
P value is for total RAS-mutated patients (both KRAS and NRAS).
Therapy was categorized into 4 groups. BSC/CR includes supportive care or cytoreductive therapy using hydroxyurea or oral etoposide. HMA include 5-azacitidine–based or decitabine-based therapy. AML-like induction therapy includes a regimen such as idarubicin and cytarabine combination. Others include immunomodulatory agents such as lenalidomide or thalidomide and TKIs (iMiDs/TKIs).
Time from initial chemotherapy or radiation treatment to t-CMML.
The median latency to diagnosis of t-CMML was 6 years (range: 1-32) (Table 1). The most common primary cancers in the t-CMML group were lymphoid malignancies (37%), followed by prostate cancer (31%) and breast cancer (13%). None of the patients in t-CMML cohort had prior history of antecedent myeloid diseases. Fifteen (38%) patients were exposed to radiation therapy alone, whereas 24 (62%) were exposed to chemotherapy or combined modality therapy.14 Cytogenetic risk and CPSS risk showed a similar pattern between the radiation therapy alone and chemotherapy or combined modality therapy groups (supplemental Tables 1 and 2), and overall survival (OS) was similar between the 2 groups (P = .428) (supplemental Figure 3).
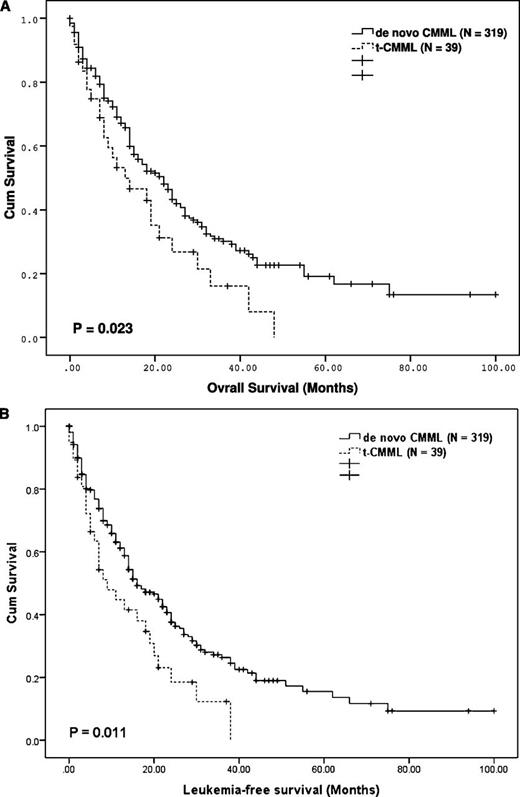
The median OS and leukemia-free survival (LFS) of the t-CMML patients (13 months [confidence interval, CI: 2.8-23.2] and 9 months [CI: 14.5-13.5], respectively) were significantly shorter than those of the de novo CMML patients (22 months [95% CI: 17.7-26.3] and 16 months [95% CI: 12.2-19.8], respectively; P = .023 and 0.011, respectively) (Figure 1). Survival differences by the therapy groups are shown in supplemental Figure 4.
Survival estimate for t-CMML and de novo CMML. Differences in OS duration (A) and LFS duration (B) between 319 patients with de novo CMML and 39 patients with t-CMML.
Survival estimate for t-CMML and de novo CMML. Differences in OS duration (A) and LFS duration (B) between 319 patients with de novo CMML and 39 patients with t-CMML.
Supplemental Table 3 shows the result of the univariate analyses for OS and LFS in the study group. The following variables were fitted into a multivariate Cox proportional hazard model: red blood cell transfusion dependency, WHO classification, CMML-specific cytogenetic risk, LDH, WBC, SCT, categorized therapies, and t-CMML. After adjustment for the prognostic effects of these covariates, t-CMML had no significant impact on OS or LFS (supplemental Table 4). The significantly shorter OS and LFS durations of the t-CMML patients in the univariate analyses were likely driven by high-risk cytogenetic abnormalities. In fact, when we removed the cytogenetic risk variable from the multivariate model, t-CMML showed statistically significant prognostic impact on OS and LFS (data not shown).
To the best of our knowledge, this is the first systematic study to show the clinical characteristics and outcomes of a reasonably large number of t-CMML patients treated at single institution. The results of the present study showed that although the groups have similar demographics and hematologic and molecular abnormality profiles, t-CMML patients possess more high-risk cytogenetic abnormalities than de novo CMML patients do. This is somewhat similar to what has been observed in patients with therapy-related AML or MDS, in whom a high incidence of poor-risk cytogenetic abnormalities is the distinguishing feature from de novo disease. In fact, OS of t-CMML was similar to that of therapy-related MDS patients who were treated at our institution during the same period (supplemental Figure 5).
In their literature review, Ahmed et al identified 8 t-CMML patients.3 Of the 5 patients who underwent cytogenetic testing, 4 carried the high-risk karyotype, and the median latency to t-CMML was 7 years (range: 2-14). These results are essentially consistent with the present study’s findings. The incidence of t-CMML in our analysis of more than 350 CMML patients was 11%, a number that perhaps reflects significant institutional bias as our institution is a tertiary cancer center.
Our multivariate model showed that HMA treatment was associated with better OS and LFS in CMML patients (supplemental Table 4). Subgroup analysis in the t-CMML cohort suggested that survival benefit by HMA treatment may be more evident in t-CMML patients (supplemental Figure 4). These findings need further validation in a larger cohort.
In summary, our findings indicated that patients can develop t-CMML 6 to 7 years after exposure to cytotoxic chemotherapy or ionizing radiation. t-CMML is associated with higher-risk cytogenetic abnormalities that manifest as poor outcomes. A large-scale multicenter study is warranted to confirm our findings. We propose that this rare disease be recognized as a new entity of therapy-related myeloid neoplasms.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors would like to thank Joseph Munch for his critical editing of the manuscript and Prof Akifumi Takaori at Kyoto University for providing significant intellectual input to the project.
This work was supported by the Ruth and Arnold Fund (G.G.-M.), the Edward P. Evans foundation (G.G.-M.), the Celgene Future Leaders in Hematology Award (K.T.), the Kimberly Patterson Leukemia Fellowship (K.T.), and in part by the National Institutes of Health through Anderson’s Cancer Center Support Grant CA016672.
Authorship
Contribution: K.T. designed the study, collected data, analyzed data, and wrote the manuscript; N.P. treated patients and wrote the manuscript; P.S. collected data and approved the manuscript; G.N.-G. and J.N. analyzed the data and wrote the manuscript; R.L. conducted molecular analyses and approved the manuscript; C.B.-R. conducted pathological diagnosis and approved the manuscript; S.P. collected data and approved the manuscript; J.C. and H.K. treated patients, provided significant intellectual input, and approved the manuscript; and G.G.-M. designed the study, guided the project, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Guillermo Garcia-Manero, Department of Leukemia, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Box 428, Houston, TX 77030; e-mail: ggarciam@mdanderson.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal