Case presentation
A 24-year-old medical editor presented to her primary care physician with fevers, epigastric pain, nausea, and vomiting upon returning from a business trip in Brazil in 2001. Symptoms resolved, but a few weeks later, she developed episodic but persistent severe central epigastric pain, accompanied by nausea and vomiting but without fever. Gastroenterological evaluation that included 2 esophagogastroduodenoscopies, a computerized tomography scan of the abdomen and pelvis, and routine testing for infectious etiologies was unrevealing. In 2002, pallor on physical examination prompted evaluation for anemia: hemoglobin was 7 g/dL, and her remaining blood counts were normal. Hemolytic indices demonstrated a markedly elevated lactic dehydrogenase (LDH) of 3998 U/L and undetectable haptoglobin in the presence of a negative direct antiglobulin test. A sugar water test was negative, but the Ham’s test was positive.
The patient was treated with prednisone, 50 mg daily, and referred to our institution for confirmation of the diagnosis. On further questioning, she reported occasional episodes of dark urine in the morning. The white blood cell count was 14 000/mm3, the neutrophil count was 11 000/mm3, hemoglobin was 8.7 g/dL, mean corpuscular volume was 112 fL, the absolute reticulocyte count was 374 000/mm3 (corresponding to 15% reticulocytes), and the platelet count was 255 000/mm3. Flow cytometry revealed that 51% of erythrocytes and 92% of granulocytes were glycosylphosphatidylinositol (GPI) negative. Total bilirubin was 2.4 mg/dL, and direct bilirubin was 0.6 mg/dL. Serum iron was 42 μg/dL with 13% saturation, and ferritin was 10 mcg/L. The bone marrow biopsy (Figure 1) is shown. Cytogenetics were normal.
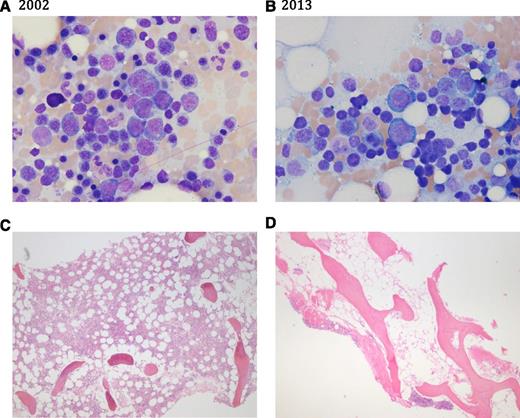
Bone marrow aspirate (A-B) and biopsy sections (C-D) in 2002 and in 2013. (A,C) Bone marrow in 2002 showing erythroid hyperplasia (myeloid to erythroid ratio 1:2), increased numbers of erythroblasts, 50% to 60% cellularity on core biopsy, and absent stainable iron. (B,D) Bone marrow in 2013 showing decreased myeloid precursors with erythroid hyperplasia, marked hypocellularity (10%), reduced megakaryocytes, and iron stain markedly increased.
Bone marrow aspirate (A-B) and biopsy sections (C-D) in 2002 and in 2013. (A,C) Bone marrow in 2002 showing erythroid hyperplasia (myeloid to erythroid ratio 1:2), increased numbers of erythroblasts, 50% to 60% cellularity on core biopsy, and absent stainable iron. (B,D) Bone marrow in 2013 showing decreased myeloid precursors with erythroid hyperplasia, marked hypocellularity (10%), reduced megakaryocytes, and iron stain markedly increased.
Over the next 3 years, she was maintained on an alternating daily regimen of 15 to 30 mg of prednisone with relief of her gastrointestinal discomfort. Hemoglobin remained 7 to 8 g/dL without transfusion; the LDH was much elevated, consistent with persistent hemolysis. She was instructed to avoid prothrombotic medications, such as oral contraceptives, and counseled to avoid pregnancy. However, in 2004, she proceeded with an unintended pregnancy, during which she suffered multiple, catastrophic complications. Hemoglobin fell to 4 to 6 g/dL with an associated rise in LDH, and she required packed red blood cell transfusions bimonthly. Thrombocytopenia appeared and worsened throughout the pregnancy to a nadir of 22 000/mm3. The pregnancy was further complicated by recurrent pyelonephritis with perinephric abscess requiring percutaneous drainage. Despite prompt introduction of prophylaxis for thrombosis with enoxaparin (1 mg/kg subcutaneously every 12 hours), she suffered a nonocclusive portal vein thrombosis, which was identified incidentally during a renal ultrasound. At 28 weeks gestation, she underwent a Cesarean section for preeclampsia, after which she was transitioned to Coumadin.
In 2005, she was entered into the SHEPHERD trial (Safety in Hemolytic PNH Patients Treated With Eculizumab: A Multi-Center Open-Label Research Design)1 and received eculizumab in dose escalation and subsequently maintained at 900 mg intravenously every 2 weeks. Transfusion requirements for packed red blood cells decreased, and the LDH normalized. In 2007, she continued to require occasional packed red blood cell transfusions to maintain hemoglobin above 7 g/dL. Prednisone was resumed in addition to eculizumab, and she was able to achieve transfusion independence. However, in 2012, she developed gradual worsening cytopenias, and by 2013, white blood cells were 2100/mm3, neutrophil count was 380/mm3, hemoglobin was 3.7 g/dL, reticulocyte count was 12 000/mm3, and platelets were 5000/mm3. Iron studies demonstrated elevated iron saturation and ferritin of 4329 mcg/L, consistent with secondary hemochromatosis. Bone marrow biopsy (Figure 1) and flow cytometry (Figure 2) are shown.
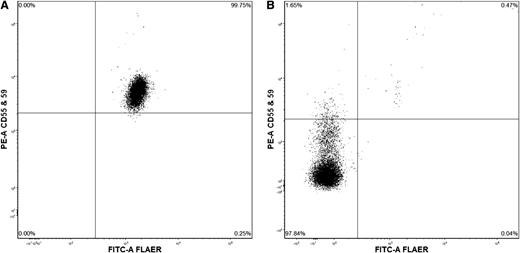
Flow cytometry of peripheral blood. (A) Normal expression. (B) Showing 97.8% granulocytes lacking staining for CD55, CD59, and fluorescein-labeled proaerolysin.
Flow cytometry of peripheral blood. (A) Normal expression. (B) Showing 97.8% granulocytes lacking staining for CD55, CD59, and fluorescein-labeled proaerolysin.
She was urged to proceed with a matched unrelated bone marrow transplantation as she lacked an HLA-matched sibling donor. However, the patient deferred transplant because of fear of its complications, as well as her ability to maintain normal life activities despite her disease. She opted for a round of immunosuppressive therapy. Horse antithymocyte globulin, cyclosporine, and eltrombopag were administered as part of a clinical trial at our institution. (This trial was registered at www.clinicaltrials.gov as #NCT01623167.) Five weeks later, her counts had improved to white blood cells of 2850/mm3, neutrophils 1550/mm3, hemoglobin 7.4 g/dL, reticulocytes 124 600/mm3, and platelets 38 000/mm3 (untransfused).
Discussion
Paroxysmal nocturnal hemoglobinuria (PNH) can be difficult to recognize not only because it is a rare disease unfamiliar to practitioners, but also because of resemblance of some of its diverse manifestations to more common syndromes. The PNH triad of intravascular hemolysis, thrombosis, and cytopenias is typically incomplete at presentation. In this patient, episodes of abdominal pain, not surprisingly, were not recognized by gastroenterologists for the esophageal spasm common with PNH; ∼10% of patients with PNH present with gastrointestinal complaints. Sequestration of nitric oxide by free hemoglobin likely triggers smooth muscle dystonia and is the cause of dysphagia, recurrent abdominal pain, and also erectile dysfunction. Investigation of anemia is more likely to suggest the diagnosis, both because of the drama of morning hemoglobinuria and because intravascular hemolysis is relatively unusual. The eponymic episodic hemoglobinuria is variably reported by patients even when questioned by the suspicious physician and present in only a minority of cases. Patients can present with thrombosis, sometimes life threatening, but usually they will show evidence of hemolysis because these 2 components of the disease are correlated. The close association of PNH with immune-mediated bone marrow failure is familiar, and patients with aplastic anemia are routinely tested for the accompanying diagnosis.
Flow cytometry utilizing antibodies to GPI-anchored proteins is now standard to establish the PNH diagnosis, replacing more cumbersome functional assays based on the characteristically increased sensitivity of PNH erythrocytes to complement.2 The well-understood acquired somatic mutation in the phosphatidylinositol glycan class A (PIG-A) gene within 1 or more hematopoietic stem cells, in combination with the more mysterious clonal expansion of these cells, leads to a mixed population of normal and mutated cells. Patients with clinical hemolysis and high risk of thrombosis have large, usually >50% granulocyte clone size. PNH is considered subclinical when the population of mutant cells is small, as occurs in aplastic anemia. Typically, the clone size remains small and stable in marrow failure, but in a few patients, it does expand over time, and PNH replaces marrow failure as the dominant phenotype.3 Analysis of the bone marrow is required to evaluate the contribution of overlapping hematologic disorders, like aplastic anemia or myelodysplastic syndrome. In this patient at presentation, a very large population of granulocytes deficient in GPI-anchored proteins and normal bone marrow cellularity (with erythroid hyperplasia) exemplified classic PNH. Over the course of a decade, the appearance of cytopenias accompanied by marrow hypocellularity signaled “reverse” evolution of the hemolytic process into hematopoietic failure.
The signs and symptoms of hemolysis usually respond within weeks of initiation of eculizumab, monoclonal antibody therapy targeting terminal complement activation. A rapid decline in LDH and corresponding rise in hemoglobin reflects inhibition of the intravascular hemolysis that is typical of eculizumab treatment. However, in this patient, anemia persisted despite normalization of LDH. Eculizumab is generally effective in hemolytic PNH, but in a few patients, anemia persists; the pathophysiology is now recognized to be exposure by eculizumab suppression of intravascular hemolysis of extravascular erythrocyte destruction.4 Risitano et al demonstrated that proximal components of the complement cascade bind PNH erythrocytes during eculizumab treatment and their markers (C3 binding) correlated with reticulocyte count.5 PNH erythrocytes coated with early complement fragments are hypothesized to be removed by macrophages in the liver and spleen, as occur with more common immune hemolytic anemias. The usefulness of corticosteroids in PNH, alone or as adjunctive therapy to eculizumab, has been difficult to establish in a disease that naturally varies in its activity and course, and there are advocates and cynics for prednisone among physicians experienced in treating PNH. The development of a monoclonal antibody that inhibits the alternative complement pathway (and thus reduces C3 deposition) might be beneficial in patients in whom extravascular hemolysis contributes to persistent anemia.6
Thrombotic events involving unusual sites (intra-abdominal and cerebral veins) are the dreaded complications of PNH, as they lead to morbidity and mortality due to organ destruction.2 The pathophysiology of thrombosis is unclear, and a number of mechanisms have been suggested, activation of PNH platelets by complement and release of thrombogenic free hemoglobin being the most compelling.7 Most experts advocate prophylaxis in PNH patients with large (>50%) clones.8 Coumadin is appropriate when the platelet count is adequate, but thrombosis may occur despite anticoagulation. Eculizumab decreases thrombotic events and can be used in patients with thrombocytopenia and does not increase the risk of bleeding.
The hypercoagulable state of pregnancy increases the risk of thrombosis in PNH and can have catastrophic outcomes for both the mother and the fetus with the risk starting in the first trimester and peaking postpartum. Despite therapeutic anticoagulation, thrombosis can be difficult to inhibit, as in this patient. Additionally, PNH poses an increased risk for infections, bleeding, anemia, and fetal loss. Pregnancy activates complement with increased terminal complex formation in the third trimester that can be further elevated in preeclampsia. Fortunately, eculizumab during pregnancy appears safe and does not harm the fetus but may require dose escalation during the third trimester.9
Subclinical hematopoietic failure, measured by sensitive assays like progenitor-derived colony formation, is present in all PNH patients. The appearance, as in our patient, of new cytopenias, relative reticulocytopenia, and reversal of iron deficiency to transfusional siderosis are indicators of the onset of frank marrow failure in PNH. Immunosuppressive therapy is often effective, and a PNH clone has been identified by some as a positive factor for likelihood of response.10 An immune pathophysiology of clonal expansion is suggested by histocompatibility antigen HLA-DR2 linkage to PNH and a skewed T-cell receptor repertoire.11,12 If a suitable donor exists, bone marrow transplantation is preferred to cure both the severe aplastic anemia and PNH.13
Acknowledgment
Funded by the Intramural Research Program of the National Institutes of Health.
Authorship
Contribution: D.M.T. and N.S.Y. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Danielle M. Townsley, National Institutes of Health, 10 Center Dr, CRC 3-5140, Bethesda, MD 20892; e-mail: Danielle.townsley@nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal