Key Points
The study reveals that circulating CLL cells contain intraclonal subgroups that differ in the level of signal-responsive surface IgM.
Subgroups with higher surface IgM express more CXCR4, potentially predicting a dangerous ability to migrate to tissue and engage antigen.
Abstract
Chronic lymphocytic leukemia (CLL) is a tumor of circulating B cells, variably stimulated and anergized following exposure to antigen in lymphoid tissues. Down-modulation of surface IgM (sIgM) occurs, but expression and signal capacity can recover in vitro and apparently in vivo during recirculation. We have now dissected individual circulating clones of CLL cases according to sIgM expression level by differential binding to bead-bound anti-IgM. Four clear subgroups (SG1-4) with increasing sIgM were identified in 37/37 cases. Engagement of sIgM induced phosphorylation of PLCγ2 and ERK1/2 at levels ranging from very low in SG1 to high in SG4. Phosphorylation was suppressed by the BTK inhibitor ibrutinib. Expression of CXCR4 also increased from SG1 to SG4, but markers of previous activation and proliferation were dominant in SG1. Incubation of whole CLL populations in vitro led to striking increases in CXCR4 expression as well as recovery of sIgM. Clonal analysis reveals dynamic SGs following presumed antigen stimulation in tissues. SG4 represents a fully recovered, potentially dangerous population equipped to migrate to tissue and receive a proliferative stimulus. SG1 likely represents a postmitotic unresponsive “resting” population. The effect of ibrutinib on the small SG4 population may be the critical factor in therapeutic success.
Introduction
Chronic lymphocytic leukemia (CLL) is a relatively indolent B-cell tumor characterized by the accumulation of long-lived cells in blood and lymphatic tissues.1 As with many mature B-cell neoplasms, expression of surface immunoglobulin (sIg) is retained.2 However, the nature of the sIg indicates that CLL is not a single disease but consists of 2 major subsets, one with unmutated (U) and the other with mutated (M) Ig variable region genes. Each subset has apparently arisen from B cells at different stages of differentiation, with U-CLL derived from a pregerminal center B cell and M-CLL from a cell that has undergone somatic hypermutation, most likely in the germinal center.2-4 The B cell of origin clearly influences subsequent tumor behavior, with U-CLL having a worse prognosis.5,6
The 2 subsets also differ in surface IgM (sIgM) density, with circulating U-CLL cells maintaining higher expression of sIgM than M-CLL.7 This is associated with functional differences, with U-CLL cells responding more than M-CLL cells to sIgM-mediated signals in vitro by phosphorylation of spleen tyrosine kinase (SYK) and mobilization of intracellular calcium (Ca2+).8,9 The likely involvement of signaling in CLL behavior has stimulated clinical trials of inhibitors of BCR-associated kinases, including SYK, BTK, or phosphoinositide 3′-kinase isoform p110δ.8,10
However, expression of functional sIgM is dynamic and reversible, with circulating CLL cells from both subsets able to recover expression if incubated in vitro.7 This is strong evidence for antigen-mediated down-regulation of sIgM in vivo. The nature of the antigens remains unclear, but the rather conserved IGV(D)J sequences, especially evident in U-CLL, suggest a limited range of antigenic structures.11-16 At least some of the same conserved IGV(D)J rearrangements in U-CLL are detectable in normal naïve B cells, with features suggestive of B cells involved in producing natural antibodies.17,18 A CD5+ fraction within the naïve B-cell population was recently suggested as a candidate source.3
Engagement of antigen is likely to occur in lymphoid proliferation centers, where multiple molecular interactions contribute to survival and proliferation.19,20 However, CLL is a leukemia with a high level of circulating tumor cells that need to access tissue sites via chemokine gradients and remain there to allow these interactions.21 Several chemokine receptors, including CXCR4, which are able to bind chemokines produced by tissue stromal cells, are highly expressed by CLL cells.22 Expression is dynamic, with down-regulation following interaction with the chemokine.21 There is also a link between sIgM engagement and reduction of expression particularly evident for CXCR4.23-25
In this study, we have identified CLL cell subgroups (SGs) within tumor clones, defined by varying levels of sIgM. The SGs ranged from one with the lowest sIgM levels having features of cells that have undergone recent activation and proliferation, to one with the highest sIgM levels, consistent with recovery of expression. High sIgM was accompanied by high expression of CXCR4, revealing a potentially dangerous minor population with migratory and signaling capacity open for a new round of proliferation. These dynamic SGs appear to reflect the phases of tumor cell recovery occurring in the peripheral blood following exit from tissue sites.
Materials and methods
CLL patients and samples
Peripheral blood samples were obtained from 37 individuals with CLL diagnosed according to the IWCLL/NCI 2008 criteria.26 Samples were collected at the University of Siena (n = 10) and at the University of Southampton (n = 27) at diagnosis and/or prior to any treatment. The study was approved by the Institutional Review Boards at the University of Southampton (228/02/t) and the University of Siena, Italy (AIRC IG-5298, ITT 2008 grants). All patients provided informed consent prior to inclusion in the study in accordance with the Declaration of Helsinki.
Peripheral blood mononuclear cells (PBMCs) were prepared and stored in liquid nitrogen. Prior to each assay, cells were thawed, washed, and allowed to recover in complete RPMI1640 medium (supplemented with 10% fetal calf serum, 2 mM glutamine, and 1 mM sodium pyruvate) for 1 hour at 37°C.26 Phenotypic, immunogenetic, fluorescence in situ hybridization, and Ca2+ mobilization studies were performed as previously described.7,27,28 The characteristics of each sample are described in supplemental Table 1 and included approximately equal numbers of U-CLL (16) and M-CLL (21).
Discrimination and investigation of CLL SGs using bead-bound anti-IgM
Dynabeads M-280 with epoxy surface groups and 2.8-µm diameter (Invitrogen, Paisley, United Kingdom) were coated with antibody according to the manufacturer’s instructions. Briefly, beads were washed in the provided C1 buffer and goat F(ab’)2 anti-human IgM or isotype control (Southern Biothech, Cambridge Biosciences, United Kingdom) was added at 10 µg of antibody/mg beads. Suspension of beads and antibody was incubated on a rotator overnight at 37°C, washed in the provided C2 buffer, and resuspended in the provided SB storage buffer at a final bead concentration of 10 mg of antibody coupled beads/mL. For long-term storage, 0.02% NaN3 was added.
PBMCs (1 × 106 cells) were incubated with anti-IgM or isotype control coated beads in 100 µL complete RPMI1640 medium for 30 minutes at 37°C (unless otherwise indicated) at a 2:1 bead:cell ratio (supplemental Material and methods; supplemental Figure 1A-B). The conversion of bead weight into bead number was calculated based on the fact that 1.5 mg of beads consists of 108 beads.
Phenotypic characterization and BCR signaling analysis was performed by flow cytometry. Acquisition, collection, and analysis with doublet discrimination were performed as described in supplemental Material and methods and supplemental Figure 2.
Flow cytometry-based single cell imaging
Samples were acquired on a 5-laser 6 channel ImageStreamX (Amnis Corp., Seattle, WA) system with a 40× magnification using INSPIRE v1.501.0 acquisition software. Cells were incubated with anti-IgM coated beads and stained with 4′,6 diamidino-2-phenylindole (DAPI) and Alexa 488-conjugated anti-CD19. DAPI was excited by a 405-nm laser (emission collected in first channel: 430-505 nm), CD19 fluorescein isothiocyanate excited by a 488-nm laser (emission collected in second channel: 505-560 nm), and DynaBeads excited by a 488-nm laser (emission collected in fourth channel 595-660 nm). Bright-field was set to channel 3 (560-595 nm) from which debris was discriminated via an area lower limit classifier of 5 µm2. A total of 100 000 images was collected and single stained controls were collected using the Comp Settings option (all channels on and bright-field/side scatter [SSC] off). Postacquisition spectral compensation and data analysis were performed using the IDEAS v5.0 image analysis software package (Amnis Corp.). Single events were identified from doublets using the area and aspect ratio on the channel 4 default mask (M04). Live B cells were defined as DAPI negative and selected for using the intensity feature on the mask complete. They were then further subdivided into those bound or not to beads (bright-field area vs bead intensity). Focused cells were identified by the bright-field gradient RMS. Differential bead binding was determined by SSC intensity on the CD19-positive population. An isotype control bound to beads was used to determine any nonspecific binding.
Phenotypic analysis of CLL SGs by immunofluorescence
The full list of antibodies used for flow cytometry is detailed in supplemental Table 2. For phenotypic characterization of surface molecules, cells bound to anti-IgM coated beads were incubated with allophycocyanin (APC)-conjugated anti-CD19 antibody and either phycoerythrin-conjugated anti-Igκ or Igλ, CD5, CD38, or CD25, or with V450-conjugated anti-CD19 and APC-conjugated anti-CXCR4. Surface staining was carried out on ice for 30 minutes in all cases.
For intracellular staining of Ki67, cells preincubated with anti-IgM coated beads and APC-conjugated anti-CD19 were fixed and permeabilized in 250 µL BD Cytofix/Cytoperm buffer for 20 minutes at 4°C. After 2 washes in 1× BD Perm/Wash buffer, cells were resuspended in a final volume of 100 µL 1× BD Perm/Wash buffer with fluorescein isothiocyanate-conjugated anti-Ki67 or isotype control antibody. Staining was performed for 30 minutes at 4°C. Acquisition and analyses were performed as described above and in the supplemental Materials and methods.
BCR signaling analysis by Phosflow
Cells were incubated with anti-IgM coated beads for 30 minutes at 37°C (activation) or on ice (background). Cells were then fixed with 1.6% paraformaldehyde for 10 minutes at room temperature and permeabilized in 90% ice-cold methanol for 10 minutes on ice. After washing, Alexa-Fluor647–conjugated anti-phosphorylated ERK1/2 (T202/Y204) or anti-phosphorylated PLCγ2 (Y759) was added for 30 minutes in the dark at room temperature.
In the inhibition assays, unlabeled cells were preincubated with 10 µM BTK inhibitor ibrutinib (PCI-32765, Stratech Scientific Ltd, Suffolk, United Kingdom) for 1 hour at 37°C. Data acquisition and analyses were performed by flow cytometry as described above. Induced phosphorylation was calculated as the difference between percentage of positive events at 37°C and that on ice.
Time course analysis of surface marker expression on whole CLL populations cultured in vitro
After thawing and recovery, cells were either subjected to phenotypic analysis (time point 0) or cultivated in 1 mL of complete RPMI1640 medium per well in a 24-well plate at 37°C at a concentration of 5 × 106 cells/mL for a maximum of 72 hours.7
At each time point, an aliquot of 1 × 106 cells was harvested from the culture and stained on ice with V450-conjugated anti-CD19, Peridinin-chlorophyll-protein-complex (PerCp)-conjugated anti-CD5 and phycoerythrin-conjugated anti-IgM (or isotype control), and APC-conjugated anti-CXCR4 (or isotype control) for 30 minutes in the dark on ice. Cells were washed twice, resuspended in 200 μL fluorescence-activated cell sorter (FACS) buffer (1% bovine serum albumin, 4 mM EDTA, and 0.15 mM NaN3 in phosphate buffered saline), and acquired on the FACS Canto II. IgM or CXCR4 expression was determined on the gated CD19+/CD5+ CLL cells and mean fluorescence intensity (MFI) was calculated at the different time points.
Statistical analyses
Categorical variables were compared by χ2 test and Fisher’s exact test when appropriate. Continuous variables were compared by Mann-Whitney nonparametric test for 2 or k independent samples. All statistical tests were 2-sided. Statistical significance was defined as P value < .05. The analysis was performed with the Statistical Package for the Social Sciences (SPSS) software v.19.0 (Chicago, IL) and Graphpad Prism 6 software (La Jolla, CA).
Results
Identification of SGs within individual CLL patients’ clones by differential binding to anti-IgM coated beads
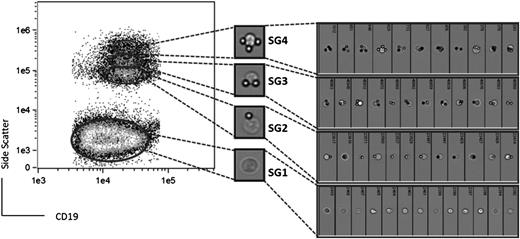
It is known that CLL cells can bind to immobilized (bead-bound) anti-IgM and that signals can be mediated.29 However, the level of sIgM within clonal CLL populations varies. We therefore probed this variability by FACS separation of cells with different numbers of attached beads based on SSC (Figure 1).
CLL clonal SGs revealed by bead-immobilized anti-sIgM separation. PBMCs were incubated either with goat F(ab’)2 anti-human IgM coated beads (A) or isotype control coated beads (B). CLL SGs were identified within the CD19+ tumor population according to different SSC in the FACS Calibur. A representative case (CLL4) is shown.
CLL clonal SGs revealed by bead-immobilized anti-sIgM separation. PBMCs were incubated either with goat F(ab’)2 anti-human IgM coated beads (A) or isotype control coated beads (B). CLL SGs were identified within the CD19+ tumor population according to different SSC in the FACS Calibur. A representative case (CLL4) is shown.
The ratio of beads/cells was selected as 2:1, because this induced a peak level of ERK1/2 phosphorylation after 30-minute exposure (supplemental Figure 1). Cells incubated with anti-IgM beads, but not with control beads, were detected as discrete subpopulations (Figure 1). We therefore decided to perform a detailed characterization of this phenomenon to investigate the potential relationship between variable levels of sIgM and biological behavior within individual malignant clones.
Characterization of CLL SGs
Typical results are shown in Figure 1A where exposure to bead-bound anti-IgM has revealed 4 clearly distinct SGs (SG1-4) within the CD19+ population. In contrast, exposure to bead-bound isotype control resulted in cells being confined to SG1 (Figure 1B). The overall percentage of CD5+ B cells among the CD19+ population was >98% in all cases and all SGs coexpressed CD5 (supplemental Figure 3), confirming that they comprised cells of the malignant clone. Among the 4 distinct SGs, SG4 exhibited the highest SSC. SG1 was always the most abundant, and the number of cells reduced sequentially in SG2-4. To assess any effect of freezing and thawing on the analysis, 3 samples were analyzed both fresh and after freezing. Although the freezing process is known to differentially affect blood cells,30 this did not alter the separation of the CLL cells into SGs, which were clearly present with a similar distribution (data not shown).
We reasoned that the individual SGs contained cells that had bound varying numbers of anti-IgM coated beads. Because the SSC of SG1 was identical to CLL cells incubated with beads coated with isotype control (Figure 1), cells of this SG are unlikely to have bound any beads, whereas SG2-4 likely contain cells with increasing number of bound beads. To confirm this, we took advantage of the autofluorescent nature of the beads (supplemental Figure 4). There was a clear increase in the autofluorescence from SG1 to SG2-4, consistent with binding to increasing numbers of anti-IgM coated beads (supplemental Figure 4).
To directly visualize bead-cell complexes, we performed ImageStreamX Flow cytometric analysis of a representative CLL sample (Figure 2). This confirmed the results obtained by studying bead autofluorescence, clearly demonstrating that cells of SG1 did not bind to anti-IgM coated beads. Moreover, the number of beads bound to cells increased with SSC (Figures 1-2). Cells of SG2, SG3, and SG4 bound, on average, 1, 2, or 3 beads, respectively. A few cells were able to bind >3 beads, but this very small population was not studied further. The ImageStream analysis also revealed that anti-IgM coated beads were not endocytosed by CLL cells (Figure 2). This observation was also confirmed in additional samples using conventional light microscopy (data not shown).
Flow cytometry-based imaging of single cells in CLL SGs. CLL SGs were identified within the live and singlet CD19+ population and selected for analysis (left) by the ImageStreamX. Random images of individual cells from CLL5 in SG1 (lower population) and the upper SGs are shown on the right.
Flow cytometry-based imaging of single cells in CLL SGs. CLL SGs were identified within the live and singlet CD19+ population and selected for analysis (left) by the ImageStreamX. Random images of individual cells from CLL5 in SG1 (lower population) and the upper SGs are shown on the right.
We performed replicate analyses of a representative sample from the same vial or from separate vials at the same time to investigate the reproducibility of SG formation. Overall, the results of replicate analysis were consistent; the SSC distribution of SG1-4 was reproducible. Also, the percentage of cells in each SG was reproducible and did not differ by >1% in an experiment with 5 replicates (data not shown). Thus, the assay can reproducibly detect both presence and distribution of SGs. However, the 30-minute incubation, which revealed the SGs, was not a point of equilibrium but was on a rising curve of binding. In fact, further incubation led to an increase in SSC and evidence for cell doublets and higher order aggregation on the forward scatter (supplemental Figure 2; data not shown). Because this was less informative, we retained the 30-minute period of incubation. However, the dynamic process means that the quantitative distribution was highly dependent on exact timing, limiting comparability when multiple samples were analyzed. Our subsequent analysis therefore focused on qualitative aspects of the phenotype and functional properties of SG1-4 within individual cases.
Analysis of sIg expression in CLL SGs
Having optimized conditions for SG analysis, we performed detailed analyses using a total of 37 CLL samples. The characteristics of CLL patients, including clinical stage, phenotypic, immunogenetic, fluorescent-in-situ-hybridization, and intracellular Ca2+ signaling results, are described in supplemental Table 1. Overall, the proportion of M- and U-CLL and the sIgM signaling properties of samples was representative of a typical cohort. SG1-4 were detected in all samples following incubation with anti-IgM coated beads.
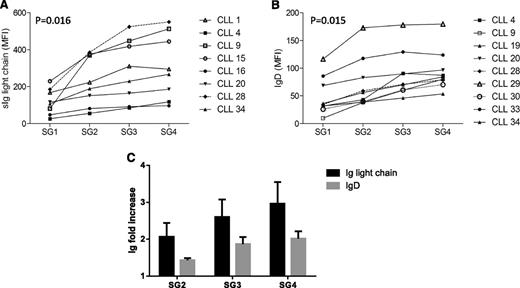
Because formation of SGs was clearly dependent on sIgM (Figure 1), we reasoned that the variable numbers of beads bound to cells in each SG reflected variation in sIgM expression levels. We were unable to directly measure sIgM expression in the SGs, because sIgM was already engaged by bead-bound anti-IgM. However, by using fluorescent antibodies, we were able to measure surface expression of monotypic light chain and sIgD. Expression of light chain significantly increased from SG1 to SG4 in all cases investigated (n = 8, 4 Igκ and 4 Igλ) (Figure 3A). There was also a significant increase in sIgD expression (Figure 3B). Clearly, the fold increase of light chain expression, which includes the increase in both sIgM and sIgD, was greater than that of sIgD (Figure 3C), demonstrating that sIgM expression increases from SG1 to SG4.
sIg light chain and IgD expression in CLL SGs. Light chain (either κ or λ) (A) or surface IgD expression (B) was analyzed within the CD19+ population on each SG in the FACS Calibur. Each line and symbol represents an individual CLL case. Values of light chain or IgD are represented as mean-fluorescence intensity on the y-axis. A nonparametric Mann-Whitney test was used to calculate the P value of the difference between SG1 and SG4. C shows the mean (± SEM) expression of Ig light chain or IgD in SG2, SG3, and SG4 relative to SG1 (normalized to 1 for each case).
sIg light chain and IgD expression in CLL SGs. Light chain (either κ or λ) (A) or surface IgD expression (B) was analyzed within the CD19+ population on each SG in the FACS Calibur. Each line and symbol represents an individual CLL case. Values of light chain or IgD are represented as mean-fluorescence intensity on the y-axis. A nonparametric Mann-Whitney test was used to calculate the P value of the difference between SG1 and SG4. C shows the mean (± SEM) expression of Ig light chain or IgD in SG2, SG3, and SG4 relative to SG1 (normalized to 1 for each case).
Functional analysis of the sIgM among the CLL SGs
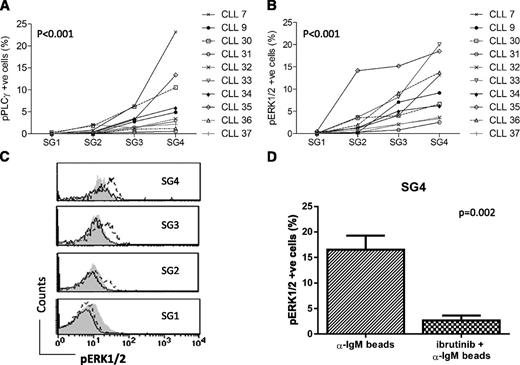
Phosphorylation of PLCγ2 and/or ERK1/2 was analyzed in specific SGs using Phosflow following stimulation of CLL cells with bead-bound anti-IgM. Increased phosphorylation was not detected in SG1 in any sample, whereas induction of PLCγ2 and/or ERK1/2 phosphorylation increased in SG2 to SG4 in 10 samples (Figure 4A-B). In general, detected levels of phosphorylated ERK1/2 were higher than phosphorylated PLCγ2, although this could reflect a more sensitive assay. However, in 2/10 samples (CLL7 and CLL30), the reverse occurred, and the significance of this dichotomy requires further investigation. Among the samples that showed increased phosphorylation of ERK1/2 in the SGs (Figure 4B), 5/10 were designated as Ca2+ “nonsignalers” when using soluble anti-IgM stimulation and applying our previous cutoff of <5% of responding cells (supplemental Table 1).7 Three of these (CLL cases 31, 32, and 37) induced only low levels of pERK1/2 even in SG4 (Figure 4B). However, 2 CLL cases (33 and 36) with weak/negative Ca2+ responses to soluble anti-IgM showed robust phosphorylation of ERK1/2 in SG3 and SG4 (Figure 4B). It appears that the use of bead-bound anti-IgM and separation into SGs can reveal responding minor cell fractions that are not evident in whole populations exposed to soluble anti-IgM.
Phosphorylation of PLCɣ and ERK1/2 induced by bead-immobilized anti-IgM in CLL SGs. Phosphorylation of PLCγ2 (pPLCγ2, A) or of ERK1/2 (pERK1/2, B) induced after exposure to bead-bound anti-IgM was measured in SGs 1 to 4 in the FACS Calibur. Percent of phosphorylated cells was calculated as the differential between the percent of cells phosphorylated at 37°C (activated) and that at ice temperature (background). (C) A representative case (CLL9) showing inhibition of pERK1/2 by ibrutinib in each individual SG. Filled histogram represents ERK1/2 phosphorylation in CLL cells incubated on ice, dashed line ERK1/2 phosphorylation at 37°C in the absence of ibrutinb, and continuous line ERK1/2 phosphorylation at 37°C in the presence of ibrutinib. (D) Effect of ibrutinib on ERK1/2 phosphorylation in SG4. Mean and SEM of the 6 cases analyzed are represented (CLL3, 7, 8, 9, 11, and 12).
Phosphorylation of PLCɣ and ERK1/2 induced by bead-immobilized anti-IgM in CLL SGs. Phosphorylation of PLCγ2 (pPLCγ2, A) or of ERK1/2 (pERK1/2, B) induced after exposure to bead-bound anti-IgM was measured in SGs 1 to 4 in the FACS Calibur. Percent of phosphorylated cells was calculated as the differential between the percent of cells phosphorylated at 37°C (activated) and that at ice temperature (background). (C) A representative case (CLL9) showing inhibition of pERK1/2 by ibrutinib in each individual SG. Filled histogram represents ERK1/2 phosphorylation in CLL cells incubated on ice, dashed line ERK1/2 phosphorylation at 37°C in the absence of ibrutinb, and continuous line ERK1/2 phosphorylation at 37°C in the presence of ibrutinib. (D) Effect of ibrutinib on ERK1/2 phosphorylation in SG4. Mean and SEM of the 6 cases analyzed are represented (CLL3, 7, 8, 9, 11, and 12).
The effect of the BTK inhibitor ibrutinib on signaling was also measured using Phosflow. Analysis focused on ERK1/2 phosphorylation, because engagement of sIgM by bead-bound anti-IgM resulted in the higher levels of phosphorylation of this kinase compared with PLCγ2 (Figure 4A-B). Ibrutinib significantly inhibited the increase in ERK1/2 phosphorylation in SG4 in 6/6 samples analyzed (P = .002). A representative case is shown in Figure 4C and cumulative data on 6 cases in Figure 4D with all showing clear inhibition. The lower levels of ERK1/2 phosphorylation induced in SG2 and 3 were also inhibited (Figure 4C).
Distribution of markers of activation and proliferation among CLL SGs
We investigated distribution of the activation markers CD38 and CD25 (IL2Rα) among SGs in 5 cases with a significant fraction of cells expressing these markers (Figure 5A-B). Expression (%) of both CD38 and CD25 was highest in SG1 and decreased in the upper SGs. In contrast, expression (MFI) of CD5, which was detected at varying levels in the 9 CLL cases analyzed (Figure 5C), showed a small increase from SG1 to SG4, although this was not statistically significant.
Surface and intracellular phenotype of CLL SGs. PBMCs were incubated with goat F(ab’)2 anti-human IgM coated beads and stained with antibodies for surface CD38 (A), CD25 (B), or CD5 (C) or for intracellular Ki67 (D) in the CLL SGs. CLL SGs were identified within the CD19+ population and the percentage of positive cells (CD38, CD25, or Ki67) or the MFI within each individual SG was determined. Analysis of CD38, CD25, and CD5 was performed using the FACS Calibur; Ki-67 used the FACS CANTO II.
Surface and intracellular phenotype of CLL SGs. PBMCs were incubated with goat F(ab’)2 anti-human IgM coated beads and stained with antibodies for surface CD38 (A), CD25 (B), or CD5 (C) or for intracellular Ki67 (D) in the CLL SGs. CLL SGs were identified within the CD19+ population and the percentage of positive cells (CD38, CD25, or Ki67) or the MFI within each individual SG was determined. Analysis of CD38, CD25, and CD5 was performed using the FACS Calibur; Ki-67 used the FACS CANTO II.
There were 4 cases where expression of the proliferation marker Ki67 in the whole population was ≥0.5%. Analysis of the distribution among SGs showed that the great majority of Ki67+ cells were in SG1 and decreased in the upper SGs. This was clearly evident in the 2 strongly Ki67+ cases (Figure 5D), where Ki67 fell from 6.6% and 5.8% of all cells in SG1 to 1.8% and 0.2% in SG4, respectively.
Expression of CXCR4 increases in the higher CLL SGs
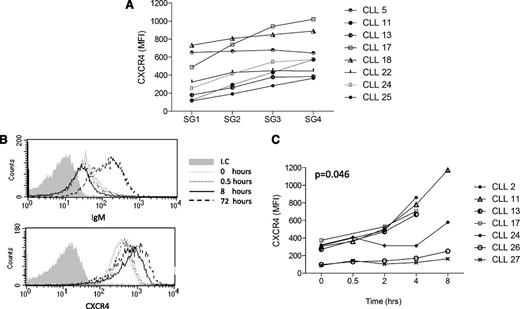
CXCR4 is expressed by all CLL cells but at variable levels.31 From analysis of CXCR4 within the whole CLL population (n = 18), MFI varied from 88 to 936 (supplemental Table 1). However, expression of CXCR4 is labile (see section below on cell recovery), with overall values tending to increase following incubation, and this was evident in the SG analysis. In terms of distribution among SGs, in 7/8 cases, the levels of CXCR4 were consistently higher in SG4 compared with SG1 (Figure 6A).
Dynamics of CXCR4 expression in CLL. CXCR4 expression was determined in SGs 1 to 4 and in the whole population using the FACS CANTO II. (A) CXCR4 expression on the CLL CD19+ cells from SG1-4. (B) expression of IgM (upper histogram) or CXCR4 (lower histogram) on CD19+/CD5+ cells from case CLL2 at different time points (0, 0.5, 8, or 72 hours) in the whole population following culture in vitro. (C) Expression of CXCR4 on the CD19+/CD5+ CLL cells at early time points (0, 0.5, 2, 4, or 8 hours) following culture in vitro. Lines indicate individual cases. I.C., isotype control at time point 0.
Dynamics of CXCR4 expression in CLL. CXCR4 expression was determined in SGs 1 to 4 and in the whole population using the FACS CANTO II. (A) CXCR4 expression on the CLL CD19+ cells from SG1-4. (B) expression of IgM (upper histogram) or CXCR4 (lower histogram) on CD19+/CD5+ cells from case CLL2 at different time points (0, 0.5, 8, or 72 hours) in the whole population following culture in vitro. (C) Expression of CXCR4 on the CD19+/CD5+ CLL cells at early time points (0, 0.5, 2, 4, or 8 hours) following culture in vitro. Lines indicate individual cases. I.C., isotype control at time point 0.
It is known that CXCR4 expression is modulated by engagement of sIgM.23-25,32 To ensure that expression of CXCR4 was unaffected by exposure to the anti-IgM coated beads for the 30-minute period of the experiment, the analysis was repeated in the presence of the SYK inhibitor R406 (Stratech Scientific Ltd., Suffolk, United Kingdom). Expression among SGs was not changed, indicating that BCR signaling had had no effect on CXCR4 expression (supplemental Figure 5).
Recovery of expression of sIgM and CXCR4 following incubation in vitro
We have previously shown that CLL cells can recover expression of sIgM during incubation for 48 to 72 hours in vitro.7 Using a case known to reexpress sIgM, we analyzed reexpression of CXCR4.33,34 We found that expression increased rapidly to a plateau level within 8 hours. Expression of sIgM did not change over this period, although it did markedly increase by 72 hours (Figure 6B). Investigation of recovered expression of sIgM or CXCR4 among SGs at this time point was not feasible due to variable levels of cell death, which could affect separation.
Analysis of whole populations in 6 further cases with variable starting levels of CXCR4 revealed a similar pattern of relatively rapid reexpression during the 8-hour period (Figure 6C). Increases in expression became evident in some cases after 2 hours, and by 8 hours, CXCR4 expression was increased in all samples. At these early time-points, recovery of IgM was not detectable, indicating a different rate of reexpression of these molecules.7
Discussion
It is now clear that the BCR provides a key to understanding the origin and behavior of CLL. There is also a therapeutic opportunity with new drugs aimed to inhibit BCR-associated pathways. The common outcome of these treatments in patients is initial lymphocytosis, with tumor cells draining from tissue sites and slowly dying in the circulation. These observations implicate both BCR signaling and migration to tissue where antigen is located as crucial factors controlling the lifestyle of CLL cells.
All tumor clones are likely to be heterogeneous and CLL is no exception. The pioneering studies with heavy water have shown that birth rates of CLL cells vary from 0.1% to >1.0% of the entire clone per day and are higher in patients with active disease.35 The proliferative stimulus appears to be engagement of the sIgM by antigen in tissue sites. The features of CLL cells suggest that cognate T-cell help is insufficient and that only a few cells proliferate. The bulk of the population appears to be driven toward anergy, characterized by a reversible down-regulation of sIgM expression.7
CLL cells in the blood represent a “shadow” of events that have occurred in the tissues. In circulating cells, there is a clear correlation between levels of sIgM expressed and the ability to signal following exposure to soluble anti-IgM in vitro.7 This is a prognostic feature and varies between U-CLL and M-CLL.36 The level of residual sIgM marks cells according to the timing of antigen encounter, the effects of such encounter on expression, and the ability to recovery and reexpress. Iterative visits to tissue sites by tumor cells protected from apoptosis are also likely. The picture in blood is the result of complex reversible kinetics and is therefore inherently unstable. However, within this population lie SGs of cells that might be most able to return to tissue sites and reengage with antigen. Inhibiting the migration and signal capacity of these SGs could be a goal for therapy.
The current analysis was aimed to find those cells using bead-bound anti-IgM, which gives a stronger, more persistent signal that occurs during the SG separation.29 Under the conditions selected, binding of beads has not reached equilibrium, and all cases show that the highest proportion of cells (SG1) had not yet bound beads and therefore does not signal. However, it revealed that a proportion of cells within each patient’s clone has bound beads stepwise with 1, 2, or 3 per cell. Even engagement of a few molecules of sIgM is apparently sufficient to mediate a signal, as was observed in mouse B cells.37 The kinetic instability makes numerical analysis difficult, but separation has allowed insight into the phenotypic and functional features of these SGs.
The SGs 1-4 expressed increasing levels of sIg light chain, as expected from higher bead-binding sIgM. However, expression of sIgD also increased from SG1 to SG4. In contrast to sIgM, sIgD expression in CLL is not down-regulated by antigen engagement, exactly as found in normal anergic B cells.38 The increased levels in the higher SGs appear to indicate a natural correlation between sIgM and sIgD expression in the B cell of origin, which becomes perturbed by antigen.
Importantly, SG4 expressed high levels of CXCR4. The relationship of this SG to that reported previously as a “resting” population, expressing increased levels of CXCR4 and low levels of CD5, is not straightforward.33,34 CD5 levels are in fact slightly raised in SG4 but are not dramatically different from other SGs. One possibility is that the sIgMhi SG4 lies within the CXCR4hi population but is a minor fraction. However, we would contend that, although “resting”, SG4 is potentially dangerous as it is open for both migration to tissue and reception of BCR signal.
The major SG is SG1, which includes the Ki67+ cells and those expressing activation markers such as CD38 and CD25. This SG appears to contain an exproliferative fraction, possibly driven into sIgMlo expression following antigen engagement. Retention of CD38 expression in SG1 temporally dissociates this molecule from the ability to respond to sIgM engagement, a clear feature of SG4.
CXCR4 is down-modulated in SG1, possibly due to previous BCR engagement,24,25,32 but this receptor is highly dynamic being also regulated by CXCL12 (SDF-1). The rapid recovery of expression in vitro is due to the recycling of the receptor39 and contrasts with the slow recovery of sIgM, which requires new biosynthesis.40 However, CXCR4 expression is associated with poor prognosis,31 and cells with high CXCR4 overexpress molecules of the NF-KB/BAFF-receptor and NOTCH1 pathways, or BCR pathway protein kinase C (PKC) family genes, all critical for proliferation and survival.34 Genes of these pathways, like BIRC3, REL2 (NF-Kb), or NOTCH1, are some of those susceptible to the genetic damage that associates with progression, resistance to chemotherapy, and/or transformation to more aggressive disease.41 Although SG4 is only a minor fraction of the CXCR4hi population, it has the highest sIgM level and is therefore most able to interact with antigen and potentially undergo proliferative events.
If the SG4 is the “dangerous” SG, it is essential to follow its progress in patients being treated with drugs aimed to inhibit BCR-associated pathways. It may be that tracking the cells with this phenotypic profile will reveal the cells to be targeted and allow probing of functional features. The BTK inhibitor ibrutinib markedly inhibits the strong bead-bound, anti-IgM–mediated signals in SG4 in vitro, predicting it will act on similar BCR-mediated pathways in vivo, which, together with potential coinhibition of other pathways, may account for clinical efficacy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Scientist Kathleen N. Potter, Manager of the Faculty of Medicine Human Tissue Bank, for her contribution to the studies in CLL.
This work was supported by Cancer Research UK and the Southampton CR-UK Centre, Ministry of University and Research-Project of National Interest 2009 (PRIN-MUR 2009, Italy), Istituto Toscano Tumori (Florence, Italy), Hairy Cell Leukemia Foundation, Inc. (Deerfield, IL), Association for International Cancer Research (United Kingdom), The Kay Kendall Leukaemia Fund, Leukaemia and Lymphoma Research, the Postgraduate National Program of José Castillejo and the Spanish Ministry of Education, the Department of Health via the National Institute for Health Research Biomedical Research Centre award to Guy's & St. Thomas' National Health Service Foundation Trust in partnership with King's College, London, and King’s College Hospital National Health Service Foundation Trust.
Authorship
Contribution: V.C. contributed to study design, performed research, analyzed and interpreted data, and wrote the manuscript; S.K., A.S., and M.S.H. performed research; P.W.J. contributed to identification and provision of well-characterized biological samples; P.S.C. performed research and analyzed data; G.P. contributed to data analysis and interpretation, manuscript writing, and critical revision; F.K.S. interpreted data, contributed to study design, and wrote the manuscript; and F.F. designed the study, identified and provided well-characterized biological samples, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Francesco Forconi, Cancer Sciences Unit, University of Southampton, Cancer Research UK Centre, Somers Cancer Research Building, MP824, Southampton General Hospital, Southampton, SO16 6YD, United Kingdom; e-mail: F.Forconi@soton.ac.uk.
References
Author notes
F.K.S. and F.F. contributed equally to this study.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal