In this issue of Blood, Perez-Garcia et al report the important discovery of an inherited germline mutation in the gene encoding the SH2B adaptor protein 3 (SH2B3) in a consanguineous family affected with autoimmune disorders and acute lymphoblastic leukemia (ALL).1
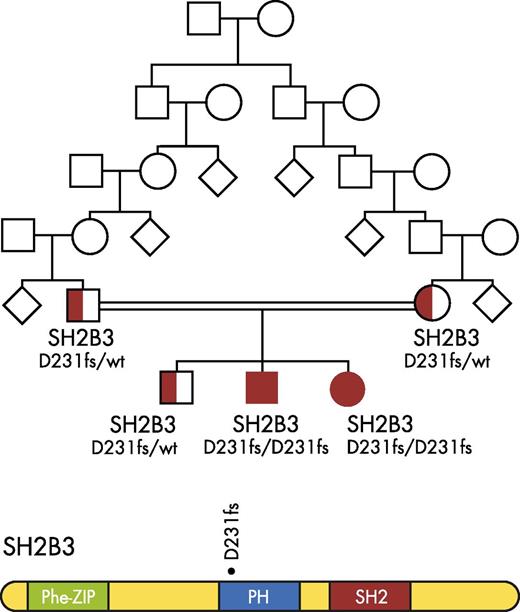
Top: Pedigree of the Eastern European family found to harbor a germline SH2B3 frameshift mutation (D231fs). Bottom: Functional domains of the SH2B3 adaptor protein: PHE-ZIP (Phenylalanine zipper); Plekstrin homology domain (Ph); SH2 (Src Homology 2 regulatory domain). The location of the germline D231fs mutation is noted. Figure is modified from Figure 1 in the article by Perez-Garcia et al that begins on page 2425.
Top: Pedigree of the Eastern European family found to harbor a germline SH2B3 frameshift mutation (D231fs). Bottom: Functional domains of the SH2B3 adaptor protein: PHE-ZIP (Phenylalanine zipper); Plekstrin homology domain (Ph); SH2 (Src Homology 2 regulatory domain). The location of the germline D231fs mutation is noted. Figure is modified from Figure 1 in the article by Perez-Garcia et al that begins on page 2425.
Both siblings with a germline homozygous frameshift mutation within the Plekstrin homology domain of SH2B3 (c.671insGGCCCCG p. Asp231Gly fs*38 [D231fs]; see figure), which led to a loss of SH2B3 protein, were affected by growth retardation, mild developmental delays, and autoimmune disorders (Hashimoto thyroiditis, autoimmune hepatitis), whereas the female sibling went on to develop B-precursor ALL at age 18 months.1 The discovery of germline mutations in SH2B3 lead to its inclusion in a small group of genes (RUNX1, NSD1, NF1, and PHF6)1 and syndromes (Down syndrome, Klinefelter syndrome, Fanconi anemia, Bloom syndrome, and ataxia-telangiectasia) associated with an inherited predisposition to ALL. These important findings suggest that SH2B3 may function as a tumor suppressor gene involved in leukemia pathogenesis.
SH2B3, originally cloned and characterized as LNK,2 encodes an adaptor protein involved in the negative regulation of several tyrosine kinases and cytokine signaling pathways (JAK, KIT, MPL, EPOR, PDGFRA/B, FLT3, FMS), which play critical roles in the development and function of hematopoietic cells.2-4 SH2B3 is induced on activation of the Janus kinase signal transducer-activator of transcription (JAK-STAT) and may function as a central negative regulatory node for multiple signaling pathways.5,6 Sh2b3-deficient mice have been shown to accumulate B-cell progenitors and have an expanded pool of hematopoietic stem cells that display increased self-renewal.3,4 In accordance with these prior findings in cell lines and animal models, it is striking that the immortalized B lymphoblastoid cell lines derived from the 2 affected siblings with homozygous SH2B3 frameshift mutations (see figure) were found to have a complete loss of SH2B3 protein, increased JAK2 and STAT3 phosphorylation, and increased growth and proliferation in vitro.1
The discovery of inherited germline mutations in SH2B3 and their link to the development of autoimmune disorders and ALL further underscores the growing biologic and clinical importance of the discovery of somatic mutations in genes regulating cytokine receptor and tyrosine kinase signaling pathways in the pathogenesis of ALL. Our collaborative team of investigators has focused on the use of comprehensive genomic technologies and next-generation sequencing to discover underlying genetic abnormalities that might serve as new therapeutic targets for high-risk forms of ALL (www.ocg.cancer.gov). Through these efforts, we7,9,10 and others8 first identified a novel subgroup of B-precursor ALL with a gene expression profile similar to Philadelphia (Ph) chromosome (BCR-ABL1)–positive ALL. Termed “Ph-like” or “BCR-ABL1–like” ALL, this subgroup constitutes 10% to 15% of pediatric and 25% of adolescent and young adult ALL cases and is associated with an extremely poor clinical outcome when treated with contemporary chemotherapy regimens. We further discovered that that Ph-like ALL is characterized by a highly heterogeneous spectrum of activating mutations or gene fusions targeting genes regulating cytokine receptor and tyrosine kinase signaling pathways (JAK1/2, ABL1/2, PDGFRB, EPOR, CSF1R, AKT2, STAT5B, CRLF2, IL7R, SH2B3), first reporting the presence of somatic mutations of SH2B3 in B-precursor ALL.10 On discovering inherited germline mutations in SH2B3, Perez-Garcia et al also went on to perform dideoxynucleotide sequencing in a cohort of 167 patients with ALL, identifying SH2B3 mutations in 2 additional patients.1 In one case, a patient with B-precursor ALL, a different homozygous frameshift mutation (c.1279insCTGTTGCCGTGTGC p.Gln427Pro fs*40), was observed in the SH2B3 Plekstrin homology domain (see figure). In the second case of T-ALL, a homozygous nonsense mutation (c.908C>A p.Ser303*) was discovered in the SH2 domain of SH2B3, accompanied by an activating NOTCH mutation. Further genomic analysis of this T-ALL case at remission recovered the wild-type SH2B3 sequence, demonstrating the likely somatic origin of this mutation.1 In our own ongoing sequencing efforts in large cohorts of high-risk pediatric patients with B-precursor ALL accrued to Children’s Oncology Group trials, we have also found that SH2B3 mutations are rare, occurring in 12 of 784 patients, a frequency of only 1.5% (Roberts et al, unpublished data).
Yet despite their rarity, the discovery of SH2B3 mutations, both somatic and germline, provide new and important insights for the continued investigation of the role of this important regulatory gene in the pathogenesis and therapy of ALL. Several highly interesting questions remain. Are homozygous mutations in SH2B3 alone sufficient to promote leukemogenesis? Although Perez-Garcia et al found that the affected sibling with a homozygous SH2B3 germline mutation and ALL also had deletions of CDKN2A, what other somatic or germline mutations were present in the leukemic cells? An unbiased sequencing study in this patient would be of great interest to identify additional potentially cooperating mutations. Indeed, in our own studies, patients with Ph-like ALL who have mutations in tyrosine kinases or genes regulating kinase signaling pathways have a spectrum of additional mutations in genes regulating B-cell development or cell growth and differentiation.10 The work of Perez-Garcia et al1 also further suggests that SH2B3 may well cooperate with other genes to promote leukemogenesis, based on their discovery of the co-association of SH2B3 and NOTCH mutations in the case of T-ALL. Indeed, in a NOTCH1-induced murine model of ALL, they found that the addition of “loss-of-function” SH2B3 mutations led to increased JAK-STAT signaling and an accelerated ALL onset.1 As Ph-like ALLs, including those with SH2B3 mutations, may be sensitive to tyrosine kinase inhibitors in vivo, or to agents inhibiting activated JAK-STAT signaling pathways, incorporating tyrosine kinase inhibitors into therapy may significantly improve clinical outcomes in these forms of ALL. Thus, these studies lay the foundation for new therapeutic approaches to the treatment of both B- and T-cell ALL cases with mutations in these pathways.
Another fascinating aspect of the study of Perez-Garcia et al1 is the observed co-association of autoimmune disorders and leukemia, underscoring the critical relationship between these diseases and the central role that SH2B3 may play in the development and regulation of critical cytokine signaling pathways that may underpin both leukemia and autoimmunity. Further investigation of the critical role of SH2B3 regulation of JAK-STAT signaling, and other cytokine signaling pathways, will likely reveal important new insights into the pathogenesis and therapeutic approach to these diseases.
Conflict-of-interest disclosure: The author declares no competing financial interests.