In this issue of Blood, Tinsley et al show that Ikaros regulates the balance between differentiation and proliferation of positively selected thymocytes. This study therefore identifies a critical role for this transcription factor in the development of a polyclonal peripheral T-cell pool.1
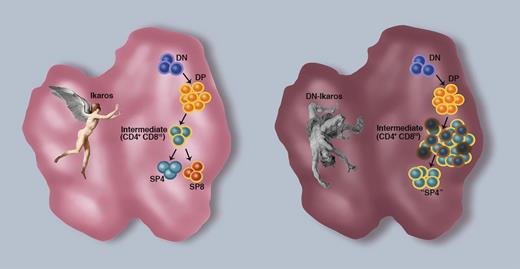
Ikaros rescue of positively selected thymocytes. T-cell differentiation requires that hematopoietic progenitors migrate from the bone marrow into the thymus. Early progenitors express neither the CD4 nor the CD8 coreceptor and are therefore termed double-negative (DN) thymocytes. On commitment to the T-cell lineage, the developing thymocytes acquire CD4 and CD8 expression and are termed double-positive (DP) thymocytes. Those DP thymocytes that are signaled via their TCR become intermediate (CD4+CD8lo) cells that can then differentiate to mature single positive CD4 (SP4) or CD8 (SP8) thymocytes. Tinsley et al1 identify Ikaros as a critical factor in the survival and differentiation of these intermediate thymocytes to a mature SP fate (left thymus). Under conditions where a dominant negative Ikaros (DN-Ikaros) is expressed, suppressing the function of all Ikaros family members, there is a concomitant proliferation and apoptosis of these intermediate thymocytes, inhibiting further differentiation and driving the clonal expansion of these semimature cells. Ikaros in flight (left thymus) and in fall (right thymus) are from Charles Paul Landon’s painting of Dedalus and Icarus (1799; Musée des Beaux-Arts et de la Dentelle, Alençon, France) and Peter Paul Rubens’ painting of The fall of Icarus (Musées Royaux Des Beaux-Arts, Brussels, Belgium), respectively. Figure design by Sandrina Kinet, Institut de Génétique Moléculaire de Montpellier, France. Professional illustration by Marie Dauenheimer.
Ikaros rescue of positively selected thymocytes. T-cell differentiation requires that hematopoietic progenitors migrate from the bone marrow into the thymus. Early progenitors express neither the CD4 nor the CD8 coreceptor and are therefore termed double-negative (DN) thymocytes. On commitment to the T-cell lineage, the developing thymocytes acquire CD4 and CD8 expression and are termed double-positive (DP) thymocytes. Those DP thymocytes that are signaled via their TCR become intermediate (CD4+CD8lo) cells that can then differentiate to mature single positive CD4 (SP4) or CD8 (SP8) thymocytes. Tinsley et al1 identify Ikaros as a critical factor in the survival and differentiation of these intermediate thymocytes to a mature SP fate (left thymus). Under conditions where a dominant negative Ikaros (DN-Ikaros) is expressed, suppressing the function of all Ikaros family members, there is a concomitant proliferation and apoptosis of these intermediate thymocytes, inhibiting further differentiation and driving the clonal expansion of these semimature cells. Ikaros in flight (left thymus) and in fall (right thymus) are from Charles Paul Landon’s painting of Dedalus and Icarus (1799; Musée des Beaux-Arts et de la Dentelle, Alençon, France) and Peter Paul Rubens’ painting of The fall of Icarus (Musées Royaux Des Beaux-Arts, Brussels, Belgium), respectively. Figure design by Sandrina Kinet, Institut de Génétique Moléculaire de Montpellier, France. Professional illustration by Marie Dauenheimer.
Ikaros is a nuclear zinc-finger transcription factor whose function is required for the normal differentiation of erythroid, myeloid, and lymphoid cells. Moreover, Ikaros also functions as a tumor suppressor, activating as well as repressing target genes and associating with chromatin remodeling complexes. Unraveling the multifaceted functions of Ikaros has been complex, at least in part because there are 7 different Ikaros isoforms with DNA-binding capacity, and these isoforms can form dimers with other family members. The members of this family include Ikaros, Aiolos, Helios, Eos, and Pegasus, with the first 3 enriched in hematopoietic tissues. As such, the effects of an Ikaros-null mutation are less pronounced than a mutant protein that also suppresses the functions of other Ikaros members.2,3
Thymopoiesis is a tightly regulated process that results in the differentiation of a nonautoreactive T-cell pool. The T-cell receptors (TCRs) expressed by these peripheral T cells must be sufficiently diverse to detect and respond to the massive number of pathogens to which an individual is exposed. Throughout life, the peripheral T-cell pool is submitted to transient fluctuations in cell numbers and subset composition, with the thymus providing an influx of new T cells. Ikaros has previously been shown to be essential for normal T-cell development, because in its absence, thymocyte differentiation is defective with a skewed ratio of CD4:CD8 T cells. The enhanced generation of mature CD4 thymocytes was initially proposed to be due to a lowered TCR signaling threshold in the absence of Ikaros.4,5
Park and colleagues1 have long been interested in thymocyte lineage choice, and in this study, they focused on the role of Ikaros in directing TCR signals into distinct developmental outcomes. To suppress the function of all Ikaros family members, they used a dominant negative (DN) approach, generating mice expressing a DN-Ikaros transgene (DN-IkTg) in all thymocyte and T-lineage cells. These mice present with a severely perturbed thymopoiesis, with a large accumulation of a CD4 thymocyte subset by ∼4 weeks of age. These thymocytes express high TCR levels, and the authors show that they have indeed undergone a TCR-dependent positive selection. However, they differ from mature single positive (SP) CD4 thymocytes that are normally exported to the periphery in that they retain heat stable antigen and lack expression of both the C-C chemokine receptor 7 (CCR7) and the interleukin 7 receptor (CD127). Using multiple approaches, the authors elegantly show that these cells represent transitional thymocytes, corresponding to CD4+CD8lo intermediate cells that are the immediate precursors of both mature CD4 and CD8 SP thymocytes. Under conditions where Ikaros isoforms cannot function, these cells do not further differentiate but rather proliferate extensively. Importantly, this uncontrolled proliferation does not result in leukemogenesis, but rather, many of these intermediate thymocytes undergo apoptosis, due to low expression of antiapoptotic genes (Bcl-2 and Mcl1). Those thymocytes that do not apoptose undergo further proliferation and expansion into an oligoclonal CD4 population. Importantly, however, they do not further differentiate and therefore represent a semimature thymocyte subset that expresses CD4 prior to undergoing commitment to a CD4 or CD8 lineage fate (see figure). Thus, the authors have identified a novel Ikaros-dependent checkpoint following TCR-dependent selection; Ikaros inhibits the aberrant proliferation of intermediate thymocytes and promotes their survival during the process of lineage choice.
Although the authors of this study focused on elucidating the mechanisms via which Ikaros regulates T-cell differentiation, their experiments also shed light on the role of Ikaros in the development of T-cell lymphoma/leukemia. Previous studies have shown a critical role for Ikaros in the development of murine T-cell lymphomas/leukemias, with 65% to 100% penetrance in mice with Ikaros mutations.6,7 However, in patients with T-cell acute lymphoblastic leukemia, Ikaros mutations are rare, accounting for <5% of cases. Ikaros mutations are, however, much more common in B-progenitor leukemias in humans,2,3,8-10 raising the question as to why the leukemic phenotypes associated with Ikaros mutations in mice and humans are disparate. The present study helps to resolve this issue because their DN-IkTg mouse is the first that does not develop spontaneous T-cell leukemias. Their model differs from previously reported mice in that, here, expression of the mutant Ikaros gene is confined to thymocytes and T-lineage cells. This would suggest that it is the ubiquitous expression of a DN-IkTg, with germ-line transmission, that promotes T-cell leukemia, whereas somatic Ikaros mutations, within hematopoietic progenitors alone, promote B-cell leukemias. This hypothesis is supported by previous experiments showing that the ubiquitous expression of a DN-IkTg results in a paradoxical increase in B-lineage differentiation in the fetal thymus.6 Furthermore, the ensemble of these studies suggests that the development of Ikaros-mutated T leukemias may occur as a result of a dysregulated epithelial-mesenchymal-endothelial cross-talk with T progenitors, under conditions where a DN Ikaros protein is expressed in all thymic compartments. The present study therefore opens many new avenues for exploring the complex Ikaros-regulated cross-talk between hematopoietic and nonhematopoietic cells, modulating lymphoid differentiation and transformation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal