Key Points
The majority of suppressive Tregs in human secondary lymphoid organs are activated, produce cytokines, and proliferate.
Human lymphoid organs may provide a platform for in vivo expansion of infused Tregs and subsequent tissue-directed homing.
Abstract
Immunomodulating regulatory T-cell (Treg) therapy is a promising strategy in autoimmunity and transplantation. However, to achieve full clinical efficacy, better understanding of in vivo human Treg biology is warranted. Here, we demonstrate that in contrast to blood and bone marrow Tregs, which showed a resting phenotype, the majority of CD4posCD25posCD127negFoxP3pos Tregs in secondary lymphoid organs were proliferating activated CD69posCD45RAneg cells with a hyperdemethylated FOXP3 gene and a broad T-cell receptor–Vβ repertoire, implying polyclonal activation. Activated CD69pos Tregs were distributed over both T-cell and B-cell areas, distant from Airepos and CD11cpos cells. In contrast to the anergic peripheral blood Tregs, lymphoid organ Tregs had significant ex vivo proliferative capacity and produced cytokines like interleukin-2, while revealing similar suppressive potential. Also, next to Treg-expressing chemokine receptors important for a prolonged stay in lymphoid organs, a significant part of the cells expressed peripheral tissue–associated, functional homing markers. In conclusion, our data suggest that human secondary lymphoid organs aid in the maintenance and regulation of Treg function and homeostasis. This knowledge may be exploited for further optimization of Treg immunotherapy, for example, by ex vivo selection of Tregs with capacity to migrate to lymphoid organs providing an in vivo platform for further Treg expansion.
Introduction
Regulatory T cells (Tregs) are critical in the maintenance of immune homeostasis. Treg-based immunotherapy is a potential treatment of many disorders, and the first human clinical trials appear promising.1-3 Still, a number of fundamental questions on human Treg biology are unanswered. Knowledge on Treg localization and phenotypes might provide clues to optimize Treg-selection, -differentiation, and -expansion protocols. To date, limited information is available on the function and phenotype of Tregs in human tissues. Mouse studies showed that in inflammatory conditions, Tregs accumulate in draining lymph nodes (LNs).4 Tregs are found in T-cell5 and B-cell areas6,7 of mouse and human lymphoid organs. In lymphoid organs of mice, Tregs suppress conventional T-cell (Tconv) priming,8,9 reduce Tconv expansion,4,10 and limit egress of Tconvs.11 Follicular Tregs suppress B-cell responses and follicular T-cell proliferation.6,7
Mouse Tregs upregulate expression of markers associated with homing to peripheral tissues following activation by dendritic cells, suggesting that Tregs migrate to the periphery to exert their function locally, similar to Tconvs.12,13 Indeed, human peripheral blood (PB) Treg-expressing homing markers for peripheral sites have been identified,14-16 and Tregs were found in human peripheral tissues in healthy steady-state conditions14,16-18 and in inflammation.19,20 Studies on adoptive transfer of Tregs with peripheral homing marker phenotypes also indicate local Treg regulation.21,22 Moreover, Treg subsets that express markers associated with T-helper (Th) counterparts were identified, and these Tregs efficiently controlled Th1 and Th223-26 responses. These studies indicate that the combination of functional characteristics and homing markers may determine optimal Treg efficacy.
Here, we examined presence, phenotype, and function of human Tregs in healthy lymphoid tissues. Interestingly, the majority of Tregs in lymphoid organs had an activated CD69posCD45RAneg and Ki67pos phenotype and a broad T-cell receptor (TCR)–Vβ repertoire. This implies that, in healthy secondary lymphoid tissues, Tregs are under constant polyclonal activation and expansion. Activated Tregs were found widely distributed in LNs and spleen (SPL), and distant from Airepos and CD11cpos cells, reducing the likelihood that these cells are activating the Tregs. While lymphoid organ Tregs showed normal suppressive capacity, they were not anergic, and produced interleukin-2 (IL-2) as well as IL-10 and interferon γ (IFN-γ). Analysis of chemokine receptor expression and migratory capacity showed that Tregs can indeed migrate to chemokines associated with peripheral tissues.
Methods
Sample source
Bone marrow (BM) and PB samples were obtained from healthy human stem cell donors, SPL samples from deceased human liver or kidney donors, liver-draining LNs (liLNs) from deceased human liver donors, and inguinal LNs (inLNs) from human kidney transplant recipients (not treated with immunosuppressive drugs) at Radboud University Medical Centre (Nijmegen, The Netherlands) and Erasmus University Medical Centre (Rotterdam, The Netherlands). PB cells for functional assays were obtained from healthy human blood donor buffy coats (Sanquin Bloodbank, Nijmegen, The Netherlands). The medical ethical committees for human research in the regions Arnhem/Nijmegen and Rotterdam approved the study. Informed consent was obtained from all study participants or their representatives in accordance with the Declaration of Helsinki.
Cell preparation
Lymphoid organ samples were forced through 74-μm netwell filters (Costar; Corning International) to obtain single-cell suspensions. Mononuclear cells were isolated by density gradient centrifugation (Lymphoprep; Nycomed Pharma). Cell subsets were obtained by positively selecting CD4pos T cells from mononuclear cell fractions by magnetic-activated cell sorting (15 μL of anti-CD4 microbeads per 107 cells; Miltenyi Biotec) where indicated, followed by staining with conjugated monoclonal antibodies (mAbs) against CD4, CD25, and CD69, and fluorescence-activated cell sorting (FACS) (Altra; Beckman Coulter). For some experiments, mononuclear cell fractions were cryopreserved prior to analysis.
Flow cytometry
Cell phenotypes were analyzed by 5-color flow cytometry (FC500; Beckman Coulter). For cell-surface staining, the following mAbs were used: CD25 (M-A251)–phycoerythrin (PE), CD29/ITGβ1 (K20)–fluorescein isothiocyanate (FITC), CD45RA (HI100)–FITC, CD127 (hIL-7R-M21)–Alexa Fluor 647, CD183/CXCR3 (1C6/CXCR3)–phycoerythrin-cyanine (PC)5, CD194/CCR4 (1G1)–PC7, CD196/CCR6 (11A9)–PE, ITGβ7 (FIB504)–PE (BD Biosciences), CD4 (SFCI12T4D11)–phycoerythrin-Texas Red (energy coupled dye [ECD]) or –PC7, CD45RA (2H4LDH11LDB9)–ECD, CD62L (DREG56)–ECD, CD69 (TP1.55.3)–PC5 or –ECD, the Beta Mark kit (Beckman Coulter), CD127 (eBioRDR5)–PC7 CD184/CXCR4 (12G5)–PC5, (eBioscience), CD197/CCR7 (150503)–PE CDw199/CCR9 (112503)–PE, CCR10 (314305)–PE (R&D Systems), CD27 (M-T271)–PE (Dako), and CD25 (4E3)–bio (Miltenyi Biotec) with Streptavidin-PC7 (eBioscience). For intracellular staining, Fix and Fix/Perm buffer (eBioscience) were used according to the manufacturer’s instructions with FoxP3 (PCH101)–FITC (eBioscience), FoxP3 (259D/C7)–Alexa Fluor 647, Helios (22F6)–Alexa Fluor 674 (Biolegend), and Ki67 (B56)–FITC (BD Biosciences). Isotype controls were used for gate settings.
In vitro activation
For analysis of proliferative capacity, 1.25 × 104 cells were cultured with or without 2.5 × 103 anti-CD3/anti-CD28 microbeads (Dynal Biotech) in the absence or presence of 12.5 U/mL IL-2 in RPMI 1640 (Invitrogen), at day 4, proliferation was measured by analyzing [3H] incorporation, as reported previously.27
For analysis of cytokine production, culture supernatants of 2.5 × 104 cells stimulated with phorbol myristate acetate (PMA) (12.5 ng/mL; Sigma-Aldrich) and ionomycin (0.5 μg/mL; Sigma-Aldrich) were collected after 24 hours, and analyzed by Luminex according to the manufacturer’s instructions (Bio-Rad). Intracellular IL-2 production was analyzed by flow cytometry as reported previously.28
Suppression
PB CD4pos T cells from 1 (allogeneneic) donor were used as responder T cells (Tresps) for all suppression assays, in combination with Tregs from different donors. Carboxyfluorescein diacetate succinimidyl ester (CFDA-SE) (Invitrogen)–labeled Tresps (2.5 × 104) were stimulated with anti-CD3/anti-CD28 microbeads (2.5 × 103) and Treg subsets were added. At day 4, 7-amino-actinomycin-D (7AAD; Sigma-Aldrich) and 1 × 104 flow count microspheres (Beckman Coulter) were added. Responder T-cell division was analyzed by flow cytometry, excluding 7AADpos cells and CFSEneg Tregs. Suppression was defined as (100 − [(numbers of proliferating Tresps cocultured with Tregs)/numbers of proliferating Tresps cultured alone) × 100%]).
Migration
CD4pos T cells (0.5-1 × 106) in XVivo15 serum-free medium (Lonza), stained with conjugated mAbs against CD25, CD127, and CD69 were added into 3-μm filter inserts (Millipore). Bottom compartments were filled with medium or medium plus CCL20/macrophage inflammatory protein-3 (MIP3a) (1 μg/mL), CCL22/macrophage-derived chemokine (MDC) (1 μg/mL), CCL25/Thymus-Expressed Chemokine (TECK) (2.5 μg/mL), or CXCL10/IFN-γ–induced protein 10 (IP-10) (1 μg/mL) (R&D Systems). Cells were harvested from bottom compartments after 2 hours, added to 104 flow count microspheres and analyzed by flow cytometry. Migration was defined as: ([numbers of migrating cells in the presence of chemokines − numbers of migrating cells in medium controls] × 100%).
FOXP3 gene methylation
Genomic DNA was isolated from human SPL-derived FACS-sorted CD4posCD25pos Tregs and CD4posCD25neg Tconvs using the QIAamp DNA Blood Mini kit (Qiagen), treated by the EpiTect Bisulfite kit (Qiagen) and amplified using bisulfite-specific polymerase chain reaction (PCR) (forward 5′ TGGATATTTGGTTAGAGTTAAGAAT 3′ and reverse 5′ ACCTAACACTCTCAAAACTTCAAAC 3′). The purified PCR product was labeled using BigDye Terminator version 1.1 Cycle Sequencing (Applied Biosystems), purified using Sephadex G-50 Fine DNA Grade (GE Healthcare), sequenced on an ABI 3130 Genetic Analyzer (Applied Biosystems), and analyzed using Sequencing Analysis version 5.4 software (Applied Biosystems).
TRB gene rearrangement
TRB rearrangements were assessed using the standardized multiplex PCR protocols (35 PCR cycles) developed by the BIOMED-2 concerted action BMH4-CT98-3936.29 The TRB complete (VDJB)–rearrangements were assessed in 2 tubes (A: 23 Vβ and 9 Jβ genes, B: 23 Vβ and 4 Jβ genes). Incomplete DJB are analyzed in 1 tube (C). Multiplex PCR products were monitored by GeneScan analysis on an ABI 3730 platform (Life Technologies) and processed by Genemapper (version 4.0) software (ABI Prism; Applied Biosystems). Interpretation of the clonality findings was performed according to the EuroClonality guidelines.30
Immunohistochemistry
Tissue samples fixed in formalin (Mallinckrodt Baker, Inc.) were embedded in paraffin and 6-μm sections were processed for immunohistochemical staining. The following antibodies were used: FoxP3(PCH101) (eBioscience), CD69(CH11) and Aire (ab78065), CD11c (EP1347Y) (Abcam). Stainings were visualized using diaminobenzidine (Thermo Scientific) or Streptavidin-biotin (LSAB Kit/AP; Dako) with permanent red (Dako) or BCIP/NBT (Vector Laboratories). Photographs taken with an Axioskop2-MOT/Axiocam-MRc5/Axiovision microscope (Zeiss) were analyzed using NIH ImageJ (version 1.45s), which allows for deconvolution of individual colors and the creation of images with overlaying pseudocolor to enhance or clarify original stainings.
Statistical analysis
Distributions of Treg and Tconv activation stages were analyzed on a log-odds scale using a mixed model. Percentages of cells positive for single markers were analyzed using a random effect logistic regression model. Migration, proliferation and cytokine production assays were analyzed using 1-way analysis of variance (ANOVA). Suppression assays were analyzed using 2-way ANOVA. P values < .05 were considered statistically significant.
Results
Different human lymphoid organs contain similar percentages of Tregs within CD4pos T-cell populations
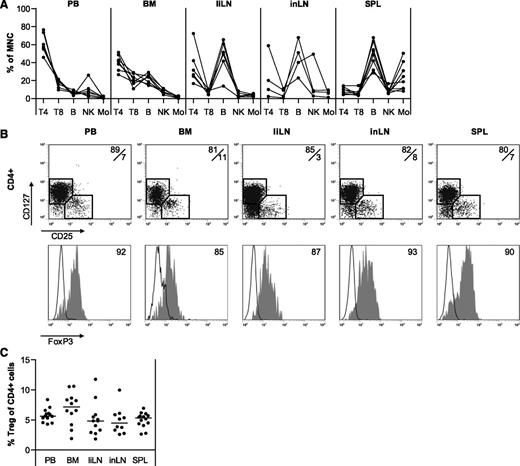
Relative compositions of human mononuclear cells in PB, BM, liLNs, inLNs, and SPL were determined (Figure 1A) by flow cytometry. As expected, PB contained a high percentage of CD4pos T cells (range, 45%-75%). Also in BM, the CD4pos T-cell fraction was predominant (range, 25%-50%). In contrast, the CD4pos T-cell content of liLNs and inLNs varied widely (range, 5%-70%) and SPL contained relatively few CD4pos T cells (range, 5%-15%). Percentages of CD25posCD127negFoxP3pos Tregs within CD4pos T cells were similar for all tissues analyzed (means, 5%-7%) (Figure 1B-C).
Distribution of mononuclear cells in different healthy human tissues. Mononuclear cells were isolated from healthy human PB, BM, liLNs, inLNs, and SPL samples and analyzed by flow cytometry. (A) Distribution of CD3posCD4pos helper T cells (T4), CD3posCD8pos T cells (T8), CD19pos B cells (B), CD56posCD3neg NK cells (NK), and CD14pos monocytes (Mo). Data are depicted as percentages of cell type within the CD45pos mononuclear gate. Each line represents 1 sample (N = 4-8 for each tissue). (B) Representative examples of CD25/CD127 staining on CD4pos T cells and FoxP3 expression on CD25posCD127neg (gray filled histograms) and CD25negCD127pos cells (black line histograms). (C) Summary of percentages of CD25posCD127neg Tregs within CD4pos T cells (N = 10-14 for each tissue). Data were compared using 1-way ANOVA and no significant differences were found.
Distribution of mononuclear cells in different healthy human tissues. Mononuclear cells were isolated from healthy human PB, BM, liLNs, inLNs, and SPL samples and analyzed by flow cytometry. (A) Distribution of CD3posCD4pos helper T cells (T4), CD3posCD8pos T cells (T8), CD19pos B cells (B), CD56posCD3neg NK cells (NK), and CD14pos monocytes (Mo). Data are depicted as percentages of cell type within the CD45pos mononuclear gate. Each line represents 1 sample (N = 4-8 for each tissue). (B) Representative examples of CD25/CD127 staining on CD4pos T cells and FoxP3 expression on CD25posCD127neg (gray filled histograms) and CD25negCD127pos cells (black line histograms). (C) Summary of percentages of CD25posCD127neg Tregs within CD4pos T cells (N = 10-14 for each tissue). Data were compared using 1-way ANOVA and no significant differences were found.
Tregs in secondary lymphoid organs are polyclonally activated
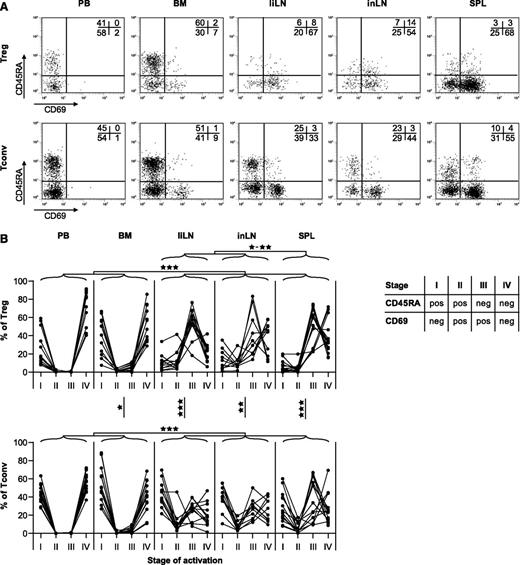
Based on surface expression of CD45RA and CD69, CD4pos T-cell activation can be divided into 4 stages15 : cells in stage I are naive (CD45RApos) and resting (CD69neg), recently activated T cells switch to a stage II CD45RAposCD69pos phenotype, and a few days later, T cells are stage III CD45RAnegCD69pos. Finally, cells turn into stage IV CD45RAnegCD69neg resting memory cells. Reactivation of a resting memory T cell causes temporary CD69 reexpression. Using this classification, we found remarkable differences between Tregs in different body compartments (Figure 2A-B). PB and BM Tregs showed similar activation patterns, with almost all Tregs in resting stages I and IV. A completely different pattern was found for Tregs in secondary lymphoid organs, where the majority of cells displayed an activated memory phenotype (stage III). Although secondary lymphoid organs also contained more activated CD4posCD25negCD127posFoxP3neg Tconvs compared with PB and BM, proportions of activated Tregs in liLNs, inLNs, and SPL were significantly higher compared with proportions of activated Tconvs.
Activation status of Tregs and Tconvs in different healthy human tissues. The activation status of human CD4pos Tregs and Tconvs as indicated by CD45RA and CD69 expression was analyzed by flow cytometry on PB, BM, liLNs, inLNs, and SPL. (A) Representative examples of CD45RA and CD69 staining on expression on CD4posCD25posCD127neg Tregs and CD4posCD25negCD127pos Tconvs. (B) Summary of distributions of Tregs and Tconvs over 4 activation stages, based on expression of CD45RA and CD69. Data are depicted as percentages of cells within each stage. Each line represents 1 sample (N = 10-14 for each tissue). Data were compared on the log-odds scale using a mixed model, and significant differences are indicated: *P < .05; **P < .01; ***P < .001.
Activation status of Tregs and Tconvs in different healthy human tissues. The activation status of human CD4pos Tregs and Tconvs as indicated by CD45RA and CD69 expression was analyzed by flow cytometry on PB, BM, liLNs, inLNs, and SPL. (A) Representative examples of CD45RA and CD69 staining on expression on CD4posCD25posCD127neg Tregs and CD4posCD25negCD127pos Tconvs. (B) Summary of distributions of Tregs and Tconvs over 4 activation stages, based on expression of CD45RA and CD69. Data are depicted as percentages of cells within each stage. Each line represents 1 sample (N = 10-14 for each tissue). Data were compared on the log-odds scale using a mixed model, and significant differences are indicated: *P < .05; **P < .01; ***P < .001.
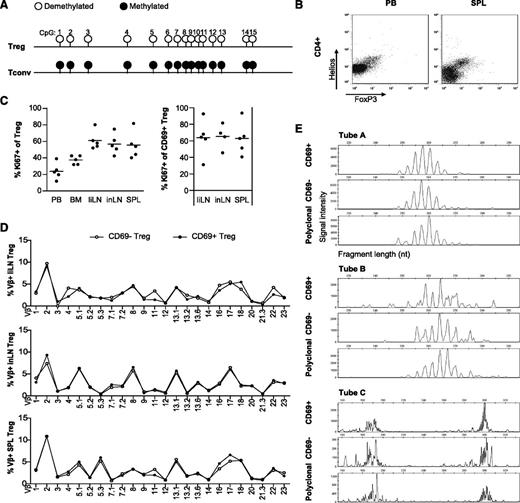
To exclude the possibility that the activated CD4posCD25posCD127negFoxP3pos cells were activated Tconvs, we demonstrated that human SPL-derived Tregs have a hyperdemethylated FOXP3 gene (Figure 3A) and coexpress Foxp3 and Helios (Figure 3B). Helios has previously been described to discriminate thymic-derived from induced Foxp3pos Tregs.31 These data support the notion that activated CD4posCD25posCD127negFoxP3pos cells in lymphoid tissues are indeed Tregs, and not activated Tconvs.
Tregs in human secondary lymphoid are in a late activation stage and are polyclonally activated. (A) CpG methylation in the Treg cell-specific demethylation region of the FOXP3 gene in FACS-sorted, human SPL-derived CD4posCD25hpos Tregs and CD4posCD25neg Tconvs. Methylation status of the indicated CpG positions is indicated by circles: ○, hyperdemethylated (<95% methylation) or ●, methylated (>95% methylation). Complete demethylation was indicated by >95% conversion of C to T in the sequenced product; sites of complete methylation were indicated when >95% of the sequence peak heights indicated C (N = 3 male donors, a representative example is shown). (B) Mononuclear cells were obtained from healthy human PB and SPL samples and analyzed for expression of Helios and FoxP3 on CD4pos cells by flow cytometry (N = 3 for each tissue, representative examples are shown). (C) Flow cytometry of Ki67 expression in human PB, BM, liLNs, inLNs, and SPL Tregs (left panel) and in CD4posCD25posCD69pos liLN, inLN, and SPL Tregs (right panel; N = 5 for each tissue). Data were analyzed using a random-effect logistic regression model and no significant differences were found. (D) Flow cytometry of TCR-Vβ expression of CD69neg and CD69pos Tregs in liLN, inLN, and SPL samples. Representative examples are shown (N = 2-6 for each tissue). (E) TCR gene rearrangement patterns of CD25posCD69pos and CD25posCD69neg sorted T cells from PB and SPL, showing polyclonal TRB gene rearrangement patterns, both the complete VDJ-rearranged TRB genes (tubes A and B) and the incomplete DJ-rearranged TRB genes (tube C). A polyclonal sample is shown as control. A representative example is shown (N = 2); duplicates revealed similar patterns (not shown).
Tregs in human secondary lymphoid are in a late activation stage and are polyclonally activated. (A) CpG methylation in the Treg cell-specific demethylation region of the FOXP3 gene in FACS-sorted, human SPL-derived CD4posCD25hpos Tregs and CD4posCD25neg Tconvs. Methylation status of the indicated CpG positions is indicated by circles: ○, hyperdemethylated (<95% methylation) or ●, methylated (>95% methylation). Complete demethylation was indicated by >95% conversion of C to T in the sequenced product; sites of complete methylation were indicated when >95% of the sequence peak heights indicated C (N = 3 male donors, a representative example is shown). (B) Mononuclear cells were obtained from healthy human PB and SPL samples and analyzed for expression of Helios and FoxP3 on CD4pos cells by flow cytometry (N = 3 for each tissue, representative examples are shown). (C) Flow cytometry of Ki67 expression in human PB, BM, liLNs, inLNs, and SPL Tregs (left panel) and in CD4posCD25posCD69pos liLN, inLN, and SPL Tregs (right panel; N = 5 for each tissue). Data were analyzed using a random-effect logistic regression model and no significant differences were found. (D) Flow cytometry of TCR-Vβ expression of CD69neg and CD69pos Tregs in liLN, inLN, and SPL samples. Representative examples are shown (N = 2-6 for each tissue). (E) TCR gene rearrangement patterns of CD25posCD69pos and CD25posCD69neg sorted T cells from PB and SPL, showing polyclonal TRB gene rearrangement patterns, both the complete VDJ-rearranged TRB genes (tubes A and B) and the incomplete DJ-rearranged TRB genes (tube C). A polyclonal sample is shown as control. A representative example is shown (N = 2); duplicates revealed similar patterns (not shown).
To further analyze the activation status of the Tregs in lymphoid organs, we determined expression of Ki67, a marker for proliferating cells. On average, 65% of CD69pos Tregs in liLNs, inLNs, and SPL were Ki67pos (Figure 3C). In validation experiments, Tregs upregulated Ki67 expression from day 2 onward upon in vitro activation, with high expression levels at day 4 (data not shown). These data suggest that the majority of CD69pos Tregs are proliferating cells in a late activation stage. The percentage of CCR7pos and CD27pos Tregs was similar in CD45RAnegCD69pos and CD45RAnegCD69neg Tregs (data not shown), suggesting no preference for activation of a specific subtype of Tregs. To see whether the activated state of lymphoid tissue Tregs was due to specific antigenic stimulation resulting in monoclonal or oligoclonal proliferation, or rather due to polyclonal activation, TCR-Vβ patterns of CD69pos and CD69neg Tregs in inLNs, liLNs, and SPL samples were analyzed (Figure 3D). While TCR-Vβ patterns within each tissue varied between donors (data not shown), for each donor, the activated and resting Tregs in secondary lymphoid tissues showed a similar and broad TCR-Vβ pattern. The polyclonal nature of the TCR-Vβ repertoire in FACS-sorted CD69neg and CD69pos SPL Tregs was confirmed by PCR-based TRB gene rearrangement analysis of Vβ-(Dβ)-Jβ and Dβ-Jβ, showing polyclonal rearrangement patterns (Figure 3E). Together, the patterns clearly indicate that in noninflammatory conditions, Tregs in human secondary lymphoid organs are polyclonally activated.
Activated and nonactivated human Tregs are located distantly from Airepos and CD11cpos cells
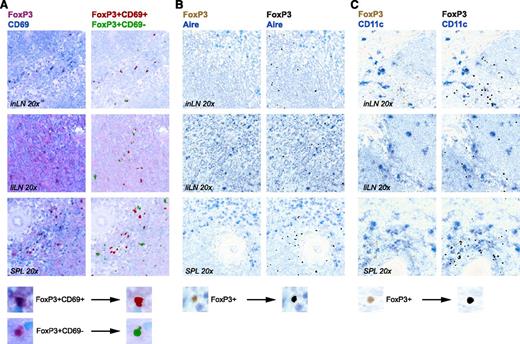
Using immunohistochemistry, we found Tregs widely distributed over T-cell and B-cell areas, confirming data from previous studies.5-7 Distributions of CD69pos and CD69neg Tregs were similar (Figure 4A). Mouse LN Airepos stromal cells are implicated in activation and deletion of autoreactive CD8pos T cells.32,33 As the repertoire of Tregs is autoantigen biased, we wondered whether we could find colocalization of Tregs and Airepos cells in human lymphoid organs. Tregs were not located in the vicinity of Airepos cells (Figure 4B). They were also not found near CD11cpos (antigen-presenting) cells (Figure 4C). These data reduce the likelihood that an interaction of Tregs with Airepos or CD11cpos cells accounts for CD69 expression on Tregs.
Immunohistochemistry of FoxP3poscells in human secondary lymphoid organs. Immunohistochemistry of representative specimens of human liLNs, inLNs, and SPL samples. (A) Anti-FoxP3 (magenta) and anti-CD69 (blue) staining (magnification, ×20). (Left panel) Photo image. (Right panel) Composite image with pseudocolor: FoxP3posCD69pos (red) and FoxP3posCD69neg (green). (B-C) Anti-FoxP3 (brown) and anti-Aire (blue) (B) or anti-CD11c (blue) (C). (Left panel) Photo image. (Right panel) Composite image with pseudocolor: FoxP3pos (black). A representative digital magnification of the composite image procedure to generate pseudocolors in ImageJ is shown on the bottom.
Immunohistochemistry of FoxP3poscells in human secondary lymphoid organs. Immunohistochemistry of representative specimens of human liLNs, inLNs, and SPL samples. (A) Anti-FoxP3 (magenta) and anti-CD69 (blue) staining (magnification, ×20). (Left panel) Photo image. (Right panel) Composite image with pseudocolor: FoxP3posCD69pos (red) and FoxP3posCD69neg (green). (B-C) Anti-FoxP3 (brown) and anti-Aire (blue) (B) or anti-CD11c (blue) (C). (Left panel) Photo image. (Right panel) Composite image with pseudocolor: FoxP3pos (black). A representative digital magnification of the composite image procedure to generate pseudocolors in ImageJ is shown on the bottom.
Activated and resting Tregs in secondary lymphoid organs show normal suppressive capacity and display increased proliferative and cytokine-producing potential
To assess the suppressive capacity of activated and nonactivated secondary lymphoid organ-derived Tregs, FACS-sorted SPL CD69pos and CD69neg Tregs and PB (CD69neg) Tregs were analyzed in suppression assays (Figure 5A). SPL CD69neg and CD69pos Tregs showed suppressive activity comparable to PB Tregs, indicating that both resting as well as activated SPL Tregs display full suppressive capacity.
CD69posand CD69negTregs in SPL and CD69negTregs in PB have similar suppressive capacity, but different proliferative and cytokine production potentials. CD4pos T cells were isolated from SPL and PB samples of healthy human donors, and sorted into CD69neg and CD69pos Tregs (SPL) or only CD69neg Tregs (PB). (A) Suppressive capacity of Treg subsets was determined in coculture assays using flow cytometry. CFSE-labeled allogeneic CD4pos Tresp isolated from PB were activated in vitro with anti-CD3/CD28 microbeads (bead:cell ratio, 1:5). Treg subsets were titrated into these cultures at indicated Tresp:Treg subset ratios and percentage of suppression is shown (N = 3). Data were compared using 2-way ANOVA and no significant differences were found between suppression capacities of Treg subsets. (B) Proliferative capacity of Treg subsets without or with anti-CD3/CD28 microbeads stimulation in the absence or presence of exogenously added IL-2. Proliferation was determined by measuring [3H]thymidine incorporation at day 4 (mean for each donor plus SD, N = 3). Data were compared using 1-way ANOVA. Significant differences are indicated: *P < .05; **P < .01; ***P < .001. (C) Treg subsets were stimulated for 24 hours with PMA and ionomycin, and culture supernatants were analyzed for the concentration of indicated cytokines by Luminex (N = 3). Data were compared using 1-way ANOVA. Significant differences are indicated: *P < .05; **P < .01; ***P < .001. (D) PB and SPL-derived CD4pos T cells were stimulated for 24 hours with PMA and ionomycin in the presence of Brefeldin A and analyzed for expression of FoxP3 and production of IL-2 by flow cytometry (N = 3 for each tissue, representative examples are shown).
CD69posand CD69negTregs in SPL and CD69negTregs in PB have similar suppressive capacity, but different proliferative and cytokine production potentials. CD4pos T cells were isolated from SPL and PB samples of healthy human donors, and sorted into CD69neg and CD69pos Tregs (SPL) or only CD69neg Tregs (PB). (A) Suppressive capacity of Treg subsets was determined in coculture assays using flow cytometry. CFSE-labeled allogeneic CD4pos Tresp isolated from PB were activated in vitro with anti-CD3/CD28 microbeads (bead:cell ratio, 1:5). Treg subsets were titrated into these cultures at indicated Tresp:Treg subset ratios and percentage of suppression is shown (N = 3). Data were compared using 2-way ANOVA and no significant differences were found between suppression capacities of Treg subsets. (B) Proliferative capacity of Treg subsets without or with anti-CD3/CD28 microbeads stimulation in the absence or presence of exogenously added IL-2. Proliferation was determined by measuring [3H]thymidine incorporation at day 4 (mean for each donor plus SD, N = 3). Data were compared using 1-way ANOVA. Significant differences are indicated: *P < .05; **P < .01; ***P < .001. (C) Treg subsets were stimulated for 24 hours with PMA and ionomycin, and culture supernatants were analyzed for the concentration of indicated cytokines by Luminex (N = 3). Data were compared using 1-way ANOVA. Significant differences are indicated: *P < .05; **P < .01; ***P < .001. (D) PB and SPL-derived CD4pos T cells were stimulated for 24 hours with PMA and ionomycin in the presence of Brefeldin A and analyzed for expression of FoxP3 and production of IL-2 by flow cytometry (N = 3 for each tissue, representative examples are shown).
Next, we determined the in vitro proliferative capacity of activated and nonactivated secondary lymphoid organ–derived Tregs. FACS-sorted SPL CD69pos and CD69neg Tregs and PB (CD69neg) Tregs were stimulated with anti-CD3/anti-CD28 microbeads in the absence or presence of exogenous IL-2. None of the Treg subsets showed in vitro proliferation in the absence of stimulation. In sharp contrast to PB Tregs, both SPL CD69pos and CD69neg Tregs clearly revealed the capacity to proliferate when stimulated in the absence of exogenously added IL-2 (Figure 5B). Proliferation of SPL Tregs was not further increased following addition of IL-2. As this could be explained if these cells produced autologous IL-2, we measured the cytokine-producing potential of Treg subsets upon stimulation. In contrast to PB Tregs, SPL CD69pos and CD69neg Tregs indeed showed significant IL-2 production (Figure 5C-D). Interestingly, SPL Tregs also produced higher levels of IFN-γ, tumor necrosis factor α (TNFα), and IL-10 (Figure 5C; the latter mainly produced by CD69pos Tregs).
These data indicate that Tregs in human secondary organs, like SPL, are not anergic to stimulation. It seems that Tregs in the splenic microenvironment use an alternative program for their activation and autocrine cytokine production.
Treg homing receptor expression patterns and migratory characteristics differ between anatomic locations
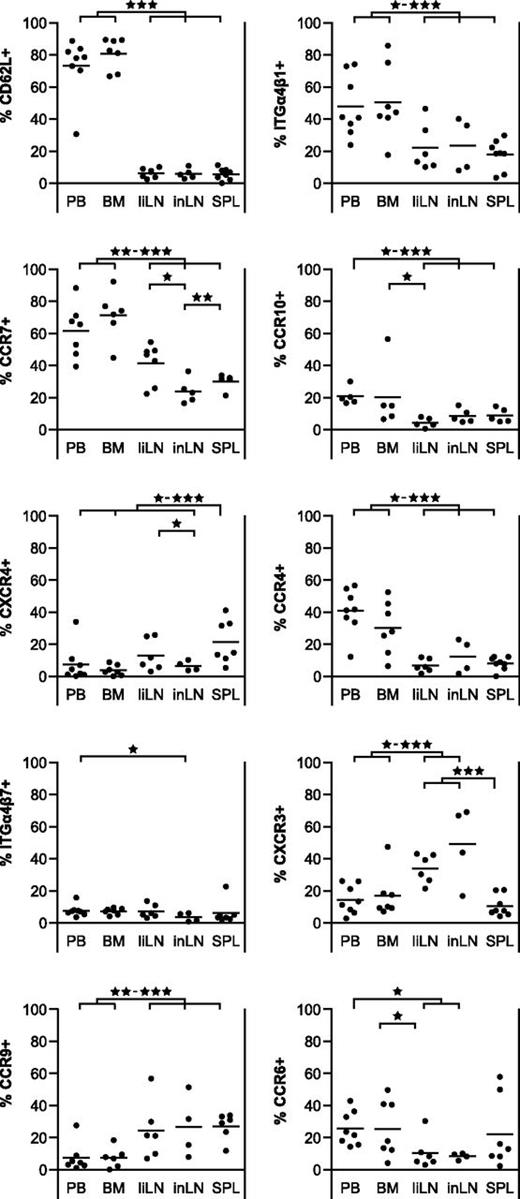
To further analyze the nature of the activation status of Tregs in secondary lymphoid tissues, we looked at homing receptor expression (Figure 6). Tregs in BM and PB revealed similar homing marker expression patterns. Of note, while Tregs in liLNs, inLNs, and SPL resembled each other with respect to homing receptor expression, their pattern was clearly different from that of PB and BM Tregs. PB and BM Tregs mainly expressed homing receptors associated with LNs (CD62L: means 70%-80%; CCR7: means 60%-70%) and skin homing (CCR4: means 30%-40%; CCR10: means 20%; ITGα4β1: means 50%), while expression of markers associated with BM (CXCR4: means < 10%) and gut homing (CCR9: means < 10%; ITGα4β7: means < 10%) were low. In contrast, Tregs in liLNs, inLNs, and SPL showed expression of gut homing–associated marker CCR9 (means 25%-30%), although there was low expression of ITGα4β7 (means < 10%). Expression of skin homing receptors was lower on liLNs, inLNs, and SPL Tregs as compared with PB and BM Tregs (means CCR4: 5%-15%; CCR10: means < 10%; ITGα4β1: means 15%-25%). In contrast to PB Tregs, CD62L expression was low on liLN, inLN, and SPL Tregs (means < 10%) and although significant portions of liLN, inLN, and SPL Tregs expressed LN homing receptor CCR7 (means 25%-40%), the percentage of CCR7pos Tregs was lower than in PB and BM Tregs. Like PB Tregs, low percentages of Tregs in secondary lymphoid organs expressed BM homing marker CXCR4 (means 5%-20%). Although the markers described are strongly associated with cell trafficking, ITGα4β1 also displays costimulatory activity.34
Expression of homing markers on Tregs in PB and BM differs from those of Tregs in secondary lymphoid organs. Flow cytometry of homing marker expression on CD4posCD25pos Tregs form human PB, BM, liLNs, inLNs, and SPL samples. Percentages of CD4posCD25pos Treg positive for indicated markers are shown (N = 4-8 for each tissue). Data were analyzed using a random-effect logistic regression model. Significant differences are indicated: *P < .05; **P < .01; ***P < .001.
Expression of homing markers on Tregs in PB and BM differs from those of Tregs in secondary lymphoid organs. Flow cytometry of homing marker expression on CD4posCD25pos Tregs form human PB, BM, liLNs, inLNs, and SPL samples. Percentages of CD4posCD25pos Treg positive for indicated markers are shown (N = 4-8 for each tissue). Data were analyzed using a random-effect logistic regression model. Significant differences are indicated: *P < .05; **P < .01; ***P < .001.
Besides homing markers associated with specific anatomic sites, as described in the previous paragraph, there are also chemokine receptors associated with specific immune functions, such as CXCR3, CCR4, and CCR6. Expression of these markers would allow Tregs to home to the same sites as Th1, Th2, and Th17 cells, respectively. Analysis revealed that PB and BM Tregs showed expression of CCR4 (means 30%-40%) and CCR6 (means 25%), and lower expression of CXCR3 (means 15%-20%), while LN-derived Tregs preferentially expressed CXCR3 (means 35%-50%) in combination with lower percentages of CCR4 (means 5%-15%) and CCR6 (means < 10%). SPL Tregs showed low expression of all 3 markers, except for a few samples with significant CCR6 expression (CXCR3: mean < 10%; CCR4: mean < 10%; CCR6: mean 20%).
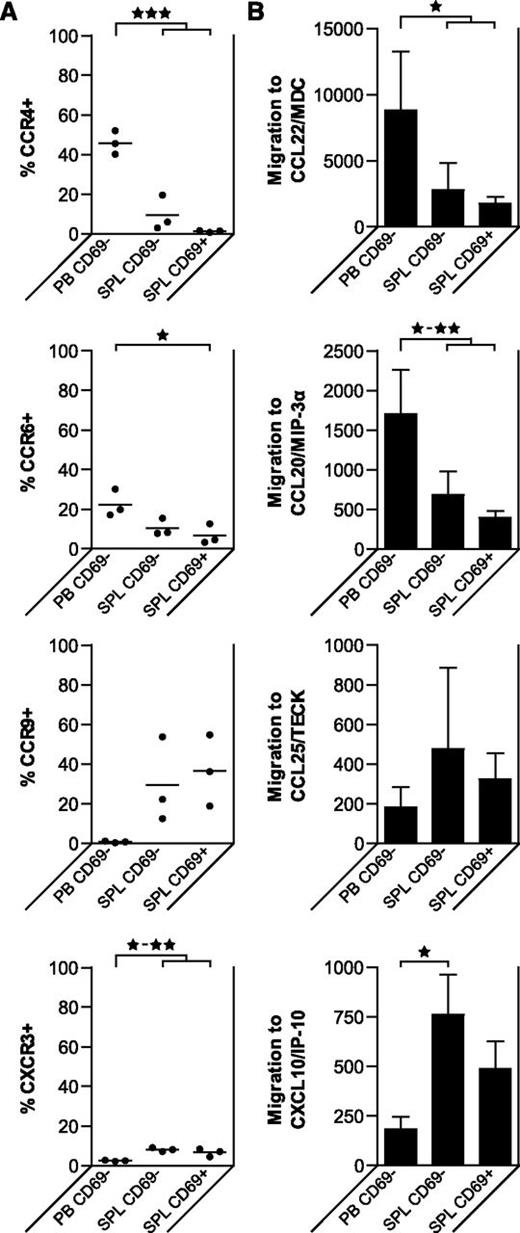
To assess whether the differences in homing receptor expression patterns of PB and BM Tregs vs those of liLN, inLN, and SPL Tregs have functional implications, we analyzed PB Tregs and SPL CD69neg and CD69pos Treg subsets with regard to their expression of selected chemokine receptors and their migration to corresponding chemokines in vitro (Figure 7). Expression of chemokine receptors CCR4, CCR6, CCR9, and CXCR3 was similar on SPL CD69neg and CD69pos Tregs. The migratory capacity toward CCL20/MIP-3α corresponded to the expression of its receptor CCR6, with high migration of PB Tregs, and lower migration of SPL CD69neg and CD69pos Tregs. The opposite pattern was observed for CXCL10/IP-10, corresponding to CXCR3 expression. The CCR4 ligand CCL22/MDC attracted significantly more PB Tregs than SPL Tregs, matching CCR4 expression patterns. Neither PB nor SPL Tregs showed significant migration toward CCL25/TECK, although its receptor CCR9 was expressed on SPL Tregs.
Tregs derived from different tissues of healthy human donors migrate to different chemokines. Flow cytometry of homing marker expression and migratory capacity of CD4posCD25posCD127negCD69pos Tregs and CD4posCD25posCD127negCD69neg in human SPL and PB samples. (A) Percentages of Tregs positive for indicated markers are shown (N = 3 for each tissue). Data were analyzed using a random effect logistic regression model. Significant differences are indicated: *P < .05; **P < .01; ***P < .001. (B) Migration assays were performed using a transwell system, with the indicated recombinant human chemokines present in the lower compartment. The migratory capacity of the Treg subsets is shown (mean plus SD, N = 3 for each tissue). Data were compared using 1-way ANOVA. Significant differences are indicated: *P < .05; **P < .01; ***P < .001.
Tregs derived from different tissues of healthy human donors migrate to different chemokines. Flow cytometry of homing marker expression and migratory capacity of CD4posCD25posCD127negCD69pos Tregs and CD4posCD25posCD127negCD69neg in human SPL and PB samples. (A) Percentages of Tregs positive for indicated markers are shown (N = 3 for each tissue). Data were analyzed using a random effect logistic regression model. Significant differences are indicated: *P < .05; **P < .01; ***P < .001. (B) Migration assays were performed using a transwell system, with the indicated recombinant human chemokines present in the lower compartment. The migratory capacity of the Treg subsets is shown (mean plus SD, N = 3 for each tissue). Data were compared using 1-way ANOVA. Significant differences are indicated: *P < .05; **P < .01; ***P < .001.
Taken together, these data suggest that Tregs in PB and BM express receptors enabling them for migration to LNs and skin, while Tregs in liLNs, inLNs, and SPL express markers that enable intralymphoid organ migration (CCR7), and have partial expression of receptors for migration to gut (CCR9). The migration patterns found corroborate the profiles found by cell-surface homing receptor expression.
Discussion
Treg immunotherapy is a promising approach for many immunology-driven pathologies. However, many questions about human Treg biology remain unanswered. In recent years, it has become evident that Tregs exert their function in secondary lymphoid tissues as well as in the periphery. Here, we characterized Tregs in healthy human PB, BM, SPL, and LNs to find novel clues about Treg function and behavior in different tissues.
Remarkably, the majority of Tregs in LNs and SPL appeared to be activated, expressing CD69, while Tregs in PB were in a resting, CD69neg state.
Previously, activated Tregs were also found to be present in human tonsils.15 In mice it was shown that expression of CD69 prevents egress from LNs by blocking S1P1 signaling.35 Treg expression of CD69 might thus retain them in the lymphoid organs and facilitate proper function. Interestingly, we found that activated Treg populations exhibited a broad TCR-Vβ pattern, implying that activation takes place in a polyclonal fashion. As the repertoire of human Tregs is shaped toward recognition of autoantigens,36 LNs may serve as a place where Tregs interact with their cognate antigen. Support for this notion comes from a mouse study where a portion of naive CD4pos T cells was found to interact with endogenous antigens in LNs, causing temporary expression of CD69 on these cells.37 Such CD69pos T cells remained in the lymphoid organs, while CD69neg T cells migrated elsewhere. Airepos stromal cells express numerous autoantigens and are involved in thymic T-cell selection processes.38 More recently, Airepos cells have been found in various human extrathymic sites, including LNs and SPL.39 Airepos cells were already shown to activate and delete autoreactive CD8pos T cells in mouse LNs.32,33 It can be speculated that these cells are in some way involved in the activation of autoantigen-specific Tregs in secondary lymphoid organs.40 Our immunohistochemistry stainings on healthy human donor SPL and LN tissue confirmed the presence of Airepos cells in human secondary lymphoid organs; however, double staining of Aire and FoxP3 showed that Tregs were not in the proximity of Airepos cells. Double staining of FoxP3 and CD11c revealed that Tregs were not in the proximity of professional antigen-presenting cells. The question thus remains which cells activate these Tregs. Our data do not exclude other (Aireneg) subsets of stromal cells as Treg activators. A candidate marker for a Treg-activating stromal cell subset might be Deaf1, as this protein was recently identified as an inducer of tissue-specific antigen expression in pancreatic LNs in a mouse model.41 Whether Deaf1 performs a similar function in human LNs remains to be established.
Uniquely, Tregs in lymphoid tissue expressed Ki67, indicating active cell cycling, which can be explained by our observation that Tregs isolated from lymphoid tissue were not anergic and could produce IL-2. Apparently, as reported for mouse Tregs,42 human Tregs are anergic in vitro, but proliferate vigorously in vivo. This, together with our finding that human Tregs in human LNs are stimulated in a polyclonal way, suggests that human LNs provide an in vivo platform for continuous activation and expansion of Tregs to sustain an immunoregulatory network.
Analysis of Treg homing marker expression and migratory capacity showed clearly that subsets of Tregs express homing markers associated with migration to peripheral tissues. PB- and BM-derived Tregs differed from those in secondary lymphoid organs. In contrast to Tregs in secondary lymphoid organs, the majority of Tregs found in PB and BM expressed both CCR7 and CD62L, homing receptors for migration toward secondary lymphoid organs. Tregs found in secondary lymphoid organs did not express CD62L, and only a fraction of these Tregs expressed CCR7, likely enabling these cells to migrate within the secondary lymphoid organs.43 Subsets of blood and BM Tregs expressed functional receptors associated with skin homing and functional receptors associated with Th2- and Th17-regulated responses, corresponding with previous reports on PB Tregs.14-16 On the other hand, Tregs in LNs and SPL expressed markers more associated with Th1-regulated responses. Tregs in these sites also showed partial expression of gut-associated homing markers (only CCR9, no integrin α4β7), although we could not show migration toward the CCR9 ligand CCL25. Expression of α4β7 on human naive and memory T cells has been shown to depend on IL-7 signaling.44 Human naive and memory CD4pos T cells express the IL-7 receptor (CD127), while in contrast, this receptor is lacking on human CD4posCD25pos Tregs.45,46 The absence of CD127 expression might explain the lack of α4β1 on Tregs. In a previous study, activated Tregs in chronically-inflamed human tonsils expressed different chemokine receptors as compared with resting Tregs, with associated differences in migration patterns.7 In our study, looking at a nondiseased, steady-state situation, we found no differences between resting and activated Tregs in this respect, possibly because there was no specific requirement for a location toward which activated cells should be guided at that time.
Tregs sorted from SPL, both activated as well as resting, showed clear suppressive capacity. This is in concert with the finding that Tregs isolated from human inflamed tonsils also showed strong suppressive capacity.47
In the current study, both fresh and cryopreserved samples were used for phenotypic characterization of Tregs. As there is conflicting data about whether or not cryopreservation reduces the percentage of Tregs in samples,48,49 we analyzed SPL samples both freshly after isolation as well as after at least 1 month of cryopreservation in liquid nitrogen. We found no difference in the percentage of Tregs, their activation status, or their chemokine expression pattern (supplemental Figure 1, available on the Blood Web site). For suppression, proliferation, cytokine production, and migration assays only freshly isolated cells were used, to prevent cryopreservation-induced artifacts, as previously described for migratory capacity.50 Another potential matter of concern is that liLN and SPL samples in this study were derived from deceased donors, and tissue ischemia may have caused nonspecific leukocyte (Tregs) activation. However, this seems unlikely because the inLNs, derived from living kidney transplant recipients, showed a similar Treg activation pattern.
In summary, we demonstrate that in healthy steady-state conditions, Tregs in human lymphoid organs are activated in a polyclonal way. These cells, at least temporarily, lose their anergic state and obtain the capacity for autocrine production of the T-cell growth factor IL-2 and the potential to proliferate, while maintaining their suppressive potential. Also, lymphoid tissue-derived Tregs expressed functional homing markers, enabling them to migrate to peripheral sites. These findings not only add to our understanding of Treg biology in human lymphoid organs, but even more so may also provide us with further clues about how to optimize Treg-based clinical protocols. For example, infused Tregs selected for a CCR7posCD62Lpos phenotype, and thus having the capacity to migrate to secondary lymphoid organs, may there, as supported by our observations, expand and obtain peripheral homing characteristics in vivo.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Bram van Cranenbroek and Gaby Derksen (Department of Laboratory Medicine–Medical Immunology, Radboud University Medical Centre, Nijmegen, The Netherlands) for their assistance in performing experiments.
Authorship
Contribution: J.H.P. designed the research, performed experiments and analyses of data, and wrote the paper; H.J.P.M.K. designed the research, analyzed data, and wrote the paper; E.F., H.J.T., and P.J.T.A.G. performed experiments and analyzed data; J.N.M.IJ. and N.P.M.S. contributed to the collection of samples from healthy human donors; J.K. contributed to the collection of samples from healthy human donors and wrote the paper; and I.J. designed the research, analyzed data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Irma Joosten, Department of Laboratory Medicine–Medical Immunology (Route 469), Radboud University Medical Centre, Postbox 9101, 6500 HB Nijmegen, The Netherlands; e-mail: I.Joosten@labgk.umcn.nl.

![Figure 5. CD69pos and CD69neg Tregs in SPL and CD69neg Tregs in PB have similar suppressive capacity, but different proliferative and cytokine production potentials. CD4pos T cells were isolated from SPL and PB samples of healthy human donors, and sorted into CD69neg and CD69pos Tregs (SPL) or only CD69neg Tregs (PB). (A) Suppressive capacity of Treg subsets was determined in coculture assays using flow cytometry. CFSE-labeled allogeneic CD4pos Tresp isolated from PB were activated in vitro with anti-CD3/CD28 microbeads (bead:cell ratio, 1:5). Treg subsets were titrated into these cultures at indicated Tresp:Treg subset ratios and percentage of suppression is shown (N = 3). Data were compared using 2-way ANOVA and no significant differences were found between suppression capacities of Treg subsets. (B) Proliferative capacity of Treg subsets without or with anti-CD3/CD28 microbeads stimulation in the absence or presence of exogenously added IL-2. Proliferation was determined by measuring [3H]thymidine incorporation at day 4 (mean for each donor plus SD, N = 3). Data were compared using 1-way ANOVA. Significant differences are indicated: *P < .05; **P < .01; ***P < .001. (C) Treg subsets were stimulated for 24 hours with PMA and ionomycin, and culture supernatants were analyzed for the concentration of indicated cytokines by Luminex (N = 3). Data were compared using 1-way ANOVA. Significant differences are indicated: *P < .05; **P < .01; ***P < .001. (D) PB and SPL-derived CD4pos T cells were stimulated for 24 hours with PMA and ionomycin in the presence of Brefeldin A and analyzed for expression of FoxP3 and production of IL-2 by flow cytometry (N = 3 for each tissue, representative examples are shown).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/13/10.1182_blood-2013-03-489443/4/m_2213f5.jpeg?Expires=1767743454&Signature=M0DWSnmFc5~AMD-Oowx1LAFBGgzPONp1DDJ42woALgFsxZR~h4XOW8Huh2RIchdcy60YNcVt0uVSbBrMZw-mB20I065pAAEz4aXJxPvPPGs-Mj7EDKmhEVo3fxF7~1EUITxHoYzSv5ikY5mKbtzWBk3SuaVkmLTmtOkDZFZFyuJYv3~O1OT3PKSa4zg0B5kWpVAdARlnz~O-wC~MXkwhzItSj2jZmvJXnrXK1BcXF3GTFNXCM4xZwO0okWwwakWCz54bo~qjJqsNuQzrQqtTBAlWdEWEV4I~GDxEakE6hTPdwvTbbxMKM061V81P4GwmNXp3~C2fApTY0BTSxHII5g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal