Key Points
Intact lymphatic vessels are required for structural and functional maintenance of surrounding tissues in the intestine and lymph nodes.
Abstract
To unveil the organotypic role and vulnerability of lymphatic vessels, we generated a lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1)-Cre/iDTR double-transgenic mouse and ablated LYVE-1–expressing lymphatic vessels in adult mice in a diphtheria toxin (DT)–inducible manner based on selective expression of LYVE-1 in most lymphatic vessels. Strikingly, lymphatic vessels in the small intestine and lymph nodes were rapidly ablated, but lymphatic vessels in the other organs were relatively intact at 24 hours after DT administration. Unexpectedly, LYVE-1-Cre/iDTR mice died of sepsis without visible edema at 24 and 60 hours after DT administration. The cause of death appeared to be related to acute failure of immune surveillance systems in the small intestine and draining lymph nodes. Of note, acute loss of lymphatic lacteals in intestinal villi appeared to trigger distortion of blood capillaries and the whole architecture of the villi, whereas acute loss of lymphatic vessels in lymph nodes caused dysfunction of lymph drainage and abnormal distribution of dendritic cells and macrophages. Thus, intact lymphatic vessels are required for structural and functional maintenance of surrounding tissues in an organotypic manner, at least in the intestine and lymph nodes.
Introduction
Because of their unique structure and distribution, lymphatic vessels play specialized roles in 1-way drainage of interstitial fluid to the systemic circulation for tissue fluid homeostasis and 1-way transfer of immune cells from peripheral tissue to draining lymph nodes for immune cell trafficking.1-6 Moreover, they are critical in 1-way transport of absorbed dietary fats and drugs as lacteals in the intestinal villi and provide a route for tumor dissemination in the periphery of solid tumors.1,2,6-8 Indeed, lymphatic vessels appear to have highly versatile structures and functions that intimately interact with their surrounding tissues, especially blood vessels in each organ. Like blood vessels, the structure and function of lymphatic vessels in each organ could be organotypic. However, to date, an organotypic role of lymphatic vessels in the maintenance of tissue homeostasis is only beginning to be understood.
Identification in recent decades of several key regulators and markers specific to lymphatic endothelial cells has accelerated findings about lymphatic vessel genesis, detailed structure, additional roles, and interactions with other cells and tissues.1-3,9 For example, lymphatic vessels are formed from the cardinal vein via a transdifferentiation process under Prox1 activity at least in mammals during embryonic development.2,10 Lymphatic endothelial cells in the initial lymphatic vessels have discontinuous buttonlike junctions that allow entry of fluid and certain immune cells into the vessel lumen through flaplike openings that form the primary lymphatic valves.11 Lymphatic endothelial cells in the collecting lymphatic vessels have continuous zipperlike interendothelial junctions, periendothelial smooth muscle cell layers, and valves for propelling unidirectional lymph flow without leakage.11 Furthermore, lymphatic vessels reciprocally play a dual role in inducing and resolving inflammation.12-14
Lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1) is one of the most specific and widely used lymphatic endothelial cell markers.15,16 It is first expressed in a few transdifferentiating endothelial cells from venous endothelial cells to lymphatic endothelial cells, which line the anterior cardinal vein of mice at embryonic days 9.0 to 9.5, and is currently considered the first indicator of lymphatic endothelial competence. In adults, LYVE-1 expression in larger collecting lymphatic vessels decreases but remains high in small lymphatic capillaries at each organ.11,17
Constitutive and conditional lineage ablation in vivo allows investigation of cell function in the context of the whole organism.18 The system involving the inducible diphtheria toxin receptor (iDTR), part of the heparin-binding epidermal growth factor–like growth factor, has been introduced as a useful method for conditional lineage ablation.19 Murine cells, unlike primate cells, are insensitive to diphtheria toxin (DT) because of its low affinity for rodent heparin-binding epidermal growth factor–like growth factor. Cell surface expression of DTR allows receptor-mediated endocytosis of DT, which induces rapid apoptosis of the target cell. Once introduced into a cell, even a single DT fragment is sufficient to kill the cell, making the DTR system very sensitive and specific.20 Taking advantage of these features, we generated a novel mouse model, the LYVE-1-Cre/iDTR mouse, which enables ablation of LYVE-1+ lymphatic vessels in a DT-dependent manner. With this model, we found unexpected critical roles of intestinal lacteals and lymphatic vessels in lymph nodes in the maintenance of villi structure and function and in the immune surveillance system.
Methods
Generation and care of LYVE-1-Cre/iDTR mice
LYVE-1-Cre mice,21 iDTR mice,18 C57BL/6 mice, and B6.SJL mice (Ly5.1) were purchased from The Jackson Laboratory (Bar Harbor, ME). The LYVE-1-Cre mouse has a selective enhanced green fluorescence protein expression in the nuclei of cells expressing LYVE-1.21 To globally ablate LYVE-1–expressing lymphatic vessels in a DT-dependent manner, LYVE-1-Cre mice were interbred with iDTR mice and designated as “LYVE-1-Cre/iDTR mice.” For systemic ablation of lymphatic vessels, LYVE-1-Cre/iDTR mice were given 100 ng of DT (Sigma-Aldrich) dissolved in Hanks balanced salt solution (Sigma-Aldrich) intravenously unless otherwise indicated. For local ablation of lymphatic vessels, mice were given 10 ng of DT intradermally into the left ear skin for 3 times. Littermate iDTR (designated as controls) mice were treated with DT in the same manner. All mice were fed with ad libitum access to a standard diet (Purina Mills Laboratory diet) and water. All mice were anesthetized by intramuscular injection of a combination of anesthetics (80 mg/kg of ketamine and 12 mg/kg of xylazine) before being euthanized. Animal care and experimental procedures were performed under approval from the Animal Care Committee of the Korea Advanced Institute of Science and Technology.
Statistics
Values are presented as mean ± standard deviation. Significant differences between means were determined by the nonpaired Student t test. Survival rates of mice after DT administration into control and LYVE-1-Cre/iDTR mice are shown by using a Kaplan-Meier curve, and its significant differences between the 2 groups were determined by the univariate log-rank test. Statistical significance was set at P < .05.
Detailed information of materials and methods is available in supplemental Methods (on the Blood Web site).
Results
LYVE-1-Cre/iDTR mice die without visible edema within 60 hours after DT administration
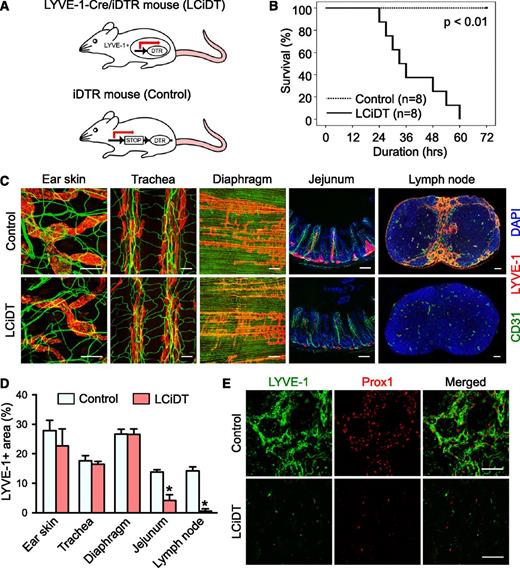
To accomplish selective ablation of lymphatic vessels in adult mice, DT (100 ng per mouse) was administered systemically into LYVE-1-Cre/iDTR mice (Figure 1A). At 24 hours after DT administration, different from our expectation, LYVE-1-Cre/iDTR mice displayed sublethal phenotypes such as markedly decreased moving activity and shivering without notable peripheral edema and erythema. Survival duration in LYVE-1-Cre/iDTR mice gradually but sharply decreased at 24 and 60 hours after DT administration, and no LYVE-1-Cre/iDTR mice had survived at 72 hours (Figure 1B). In contrast, the control mice showed no notable phenotypes or death after DT administration during the longest observation period (72 hours).
Systemic analysis of structural changes in lymphatic vessels in LYVE-1-Cre/iDTR mice after DT administration. (A) Schematic diagram of a working model of the LYVE-1-Cre/iDTR double-transgenic mouse (LCiDT) and the iDTR (control) mouse. (B) Kaplan-Meier survival curves after DT administration into control and LCiDT mice. (C) CD31+ blood vessels and LYVE-1+ lymphatic vessels of the ear skin, trachea, diaphragm, intestine, and inguinal lymph node. 4,6 Diamidino-2-phenylindole (DAPI) staining for nuclei. Scale bars, 100 μm. (D) Quantification of LYVE-1+ areas in different organs. Each group, n = 4. *P < .05. (E) LYVE-1+/Prox1+ lymphatic vessels in the submedullary region of the inguinal lymph node. Scale bars, 50 μm.
Systemic analysis of structural changes in lymphatic vessels in LYVE-1-Cre/iDTR mice after DT administration. (A) Schematic diagram of a working model of the LYVE-1-Cre/iDTR double-transgenic mouse (LCiDT) and the iDTR (control) mouse. (B) Kaplan-Meier survival curves after DT administration into control and LCiDT mice. (C) CD31+ blood vessels and LYVE-1+ lymphatic vessels of the ear skin, trachea, diaphragm, intestine, and inguinal lymph node. 4,6 Diamidino-2-phenylindole (DAPI) staining for nuclei. Scale bars, 100 μm. (D) Quantification of LYVE-1+ areas in different organs. Each group, n = 4. *P < .05. (E) LYVE-1+/Prox1+ lymphatic vessels in the submedullary region of the inguinal lymph node. Scale bars, 50 μm.
Sensitivity in the ablation of LYVE-1+ lymphatic vessels is markedly high in the small intestine and lymph nodes
To clarify a cause of the lethality of LYVE-1-Cre/iDTR mice and analyze changes in lymphatic vessels, we collected samples from the mice at 24 hours after DT administration and analyzed them in parallel with control mice. LYVE-1+ lymphatic vessels in most organs including the skin, tracheal mucosa, and diaphragm were morphologically indistinguishable between LYVE-1-Cre/iDTR mice and control mice, although the dose of DT was increased up to 500 ng (supplemental Figure 1). In contrast and quite strikingly, LYVE-1+ lacteals in the small intestine and LYVE-1+ lymphatic vessels in all lymph nodes, including the inguinal, mesenteric, axillary, and cervical lymph nodes, had mostly disappeared in LYVE-1-Cre/iDTR mice (Figure 1C-D; supplemental Figure 2). No differences between intravenous vs intraperitoneal injections were found regarding the disappearance of LYVE-1+ lymphatic vessels at the intestine and lymph nodes by DT administration into LYVE-1-Cre/iDTR mice (supplemental Figure 3). We confirmed the disappearance of lymphatic vessels by immunofluorescence staining of the lymphatic vessel markers Prox1 and VEGFR3 (Figure 1E; supplemental Figure 4). However, immunofluorescence assays in normal and LYVE-1-Cre mice revealed that degrees of LYVE-1 expression were similar in most organs and that LYVE-1 expression was confined to the lymphatic vessels except in the liver and lung (supplemental Figures 5 and 6). Moreover, the turnover rates of lymphatic endothelial cells in most organs assessed by a 5-ethynyl-2′-deoxyuridine incorporation assay22 were very low, without any differences among organs (supplemental Figure 7). No overt changes were detected in LYVE-1+ lacteals of the small intestine or LYVE-1+ lymphatic vessels of the lymph nodes in the control mice. Thus, unexpectedly, the sensitivity in ablation of LYVE-1+ lymphatic vessels in each organ differed regardless of the degrees of LYVE-1 expression and the turnover rate and was markedly high in the small intestine and lymph nodes.
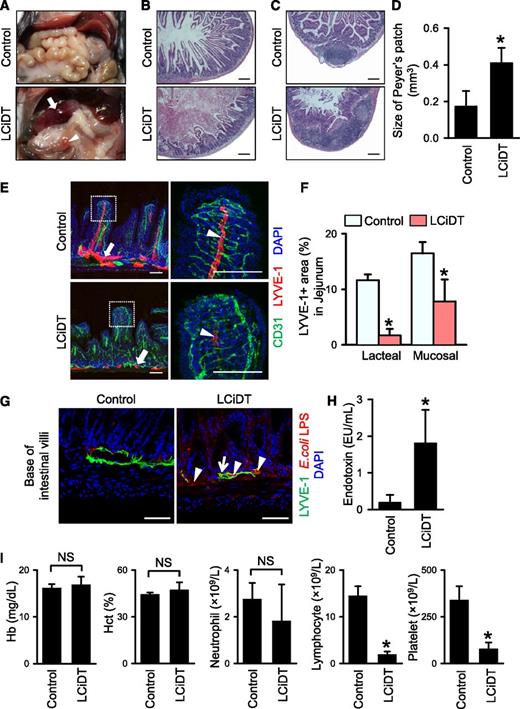
Acute ablation of LYVE-1+ lacteals causes distortion of blood capillaries and whole architecture of the villi, and leads to severe inflammation of the proximal part of the intestine and sepsis
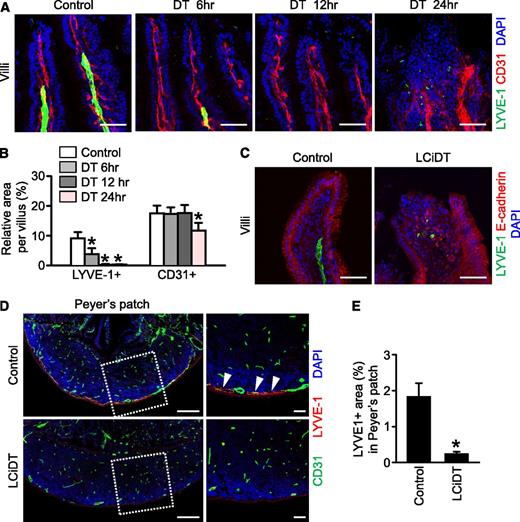
Despite the existence of a sublethal phenotype in LYVE-1-Cre/iDTR mice at 24 hours after DT administration, no overt structural differences were detected in the vital organs including the heart, lung, and kidney in either control or experimental mice (supplemental Figure 8). However, severe intestinal swelling and hemorrhage, especially at the proximal portion of the intestine, were observed in LYVE-1-Cre/iDTR mice, whereas all abdominal organs were intact in control mice (Figure 2A). Histologic analysis in LYVE-1-Cre/iDTR mice revealed swollen intestinal villi, breakage of the mucosal lining, and hematoma filling the lumen in the duodenum (Figure 2B), and hyperemic and enlarged Peyer patches were observed in the jejunum and ileum (Figure 2C-D). Detailed immunofluorescence histologic analysis indicated that LYVE-1+ lacteals were almost ablated but that LYVE-1+ mucosal lymphatic vessels were collapsed in the small intestine (Figure 2E-F) because of possible failure of absorption function. Moreover, the alignment of CD31+ blood vessels and villi was distorted (Figure 2E), and the lipopolysaccharide (LPS) derived from Escherichia coli was widely detected in the damaged villi and even inside of the mucosal lymphatic vessels in the LYVE-1-Cre/iDTR mice (Figure 2G). In parallel, common features of septic shock, which are a markedly increased (∼9.62-fold) serum endotoxin level and marked reduction of blood lymphocyte and platelet counts, were observed in LYVE-1-Cre/iDTR mice at 24 hours after DT administration (Figure 2H-I). These findings led us to examine the changes of LYVE-1+ lacteals in the LYVE-1-Cre/iDTR mice at earlier time points after DT administration. Indeed, disappearance of LYVE-1+ lacteals was evident after 6 hours, and subsequently no more LYVE-1+ lacteals were detected in the villi of the small intestine after 12 hours, whereas no overt changes were detected in the CD31+ blood vessels and villi after 6 hours, but the blood vessels were relatively enlarged after 12 hours (Figure 3A-B). These data suggest that prior damage of the LYVE-1+ lacteals could cause a distortion of the surrounding blood capillaries and the whole architecture of the villi (Figure 3A-C), therefore leading to severe inflammation of the proximal part of the small intestine and sepsis. Furthermore, the LYVE-1+ lymphatic vessels were markedly ablated at the serosal side of the enlarged Peyer patch of the LYVE-1-Cre/iDTR mice (Figure 3D-E). Peyer patches are filled with immune cells and act as regional immune sensors of the intestine.23-25
Major phenotypes of LYVE-1-Cre/iDTR mice after DT administration. Comparisons between control and LCiDT for major findings at 24 hours after DT administration. Each group, n = 4 to 5. *P < .05. (A) Gross images of the abdominal contents. The arrow indicates a hemorrhagic and swollen duodenum, and the arrowhead indicates a hyperemic and enlarged Peyer patch. (B-C) Duodenums and Peyer patches in jejunums stained with hematoxylin and eosin. Scale bars, 200 μm. (D) Quantification of sizes of Peyer patch. (E) LYVE-1+ lymphatic vessels and CD31+ blood vessels in jejunums. Indicated areas (square with white dotted line) are magnified in the right panels. The arrows indicate mucosal lymphatic vessels, and the arrowheads indicate lacteal lymphatic vessels. Scale bars, 100 μm. (F) Quantification of LYVE-1+ area in the jejunum. (G) Images showing abundant distribution of LPS derived from E coli at the base of the villi (arrowheads) and within LYVE-1+ mucosal lymphatic vessels (arrow) of the LCiDT mice at 24 hours after DT administration, whereas LPS is rarely detected in the control mice. 4,6 Diamidino-2-phenylindole (DAPI) staining for nuclei. Scale bars, 50 µm. (H) Serum endotoxin levels. (I) Blood profiles of hemoglobin, hematocrit, neutrophils, lymphocytes, and platelets. NS, not statistically significant.
Major phenotypes of LYVE-1-Cre/iDTR mice after DT administration. Comparisons between control and LCiDT for major findings at 24 hours after DT administration. Each group, n = 4 to 5. *P < .05. (A) Gross images of the abdominal contents. The arrow indicates a hemorrhagic and swollen duodenum, and the arrowhead indicates a hyperemic and enlarged Peyer patch. (B-C) Duodenums and Peyer patches in jejunums stained with hematoxylin and eosin. Scale bars, 200 μm. (D) Quantification of sizes of Peyer patch. (E) LYVE-1+ lymphatic vessels and CD31+ blood vessels in jejunums. Indicated areas (square with white dotted line) are magnified in the right panels. The arrows indicate mucosal lymphatic vessels, and the arrowheads indicate lacteal lymphatic vessels. Scale bars, 100 μm. (F) Quantification of LYVE-1+ area in the jejunum. (G) Images showing abundant distribution of LPS derived from E coli at the base of the villi (arrowheads) and within LYVE-1+ mucosal lymphatic vessels (arrow) of the LCiDT mice at 24 hours after DT administration, whereas LPS is rarely detected in the control mice. 4,6 Diamidino-2-phenylindole (DAPI) staining for nuclei. Scale bars, 50 µm. (H) Serum endotoxin levels. (I) Blood profiles of hemoglobin, hematocrit, neutrophils, lymphocytes, and platelets. NS, not statistically significant.
Damage of LYVE-1+ lacteals occurs before damage to the surrounding blood capillaries and to the whole architecture of the villi, and the lymphatic vessels in Peyer patches disappear in the LYVE-1-Cre/iDTR mice after DT administration. Control and LCiDT mice were given DT (100 ng), and their intestines were harvested at indicated times and analyzed. (A) Images showing LYVE-1+ lacteals and CD31+ blood vessels in the villi of the jejunum at 6, 12, and 24 hours after DT administration. (B) Quantification of the LYVE-1+ area and the CD31+ area in the intestinal villi. Each group, n = 4. *P < .05. (C) Images showing LYVE-1+ lacteals and E-cadherin+ epithelial cells in the intestinal villi at 24 hours after DT administration. (D) Images showing LYVE-1+ lymphatic vessels and CD31+ blood vessels in the Peyer patches. Indicated areas (square with white dotted line) are magnified in the right panel. The arrowhead indicates lymphatic vessels located at the serosal side of the Peyer patch. (E) Quantification of the LYVE-1+ area in the Peyer patches. Each group, n = 4. *P < .05. All images were stained with 4,6 diamidino-2-phenylindole (DAPI) for nuclei imaging. Scale bars, 50 µm.
Damage of LYVE-1+ lacteals occurs before damage to the surrounding blood capillaries and to the whole architecture of the villi, and the lymphatic vessels in Peyer patches disappear in the LYVE-1-Cre/iDTR mice after DT administration. Control and LCiDT mice were given DT (100 ng), and their intestines were harvested at indicated times and analyzed. (A) Images showing LYVE-1+ lacteals and CD31+ blood vessels in the villi of the jejunum at 6, 12, and 24 hours after DT administration. (B) Quantification of the LYVE-1+ area and the CD31+ area in the intestinal villi. Each group, n = 4. *P < .05. (C) Images showing LYVE-1+ lacteals and E-cadherin+ epithelial cells in the intestinal villi at 24 hours after DT administration. (D) Images showing LYVE-1+ lymphatic vessels and CD31+ blood vessels in the Peyer patches. Indicated areas (square with white dotted line) are magnified in the right panel. The arrowhead indicates lymphatic vessels located at the serosal side of the Peyer patch. (E) Quantification of the LYVE-1+ area in the Peyer patches. Each group, n = 4. *P < .05. All images were stained with 4,6 diamidino-2-phenylindole (DAPI) for nuclei imaging. Scale bars, 50 µm.
Considering that the lymphatic vessels in Peyer patches are responsible for egression of the lymphocytes and dendritic cells to the mesenteric lymph nodes via the connecting lymphatic vessels,24,25 we further examined the changes of immune cell distributions by immunohistochemical analysis. Compared with control mice, in LYVE-1-Cre/iDTR mice, CD11c+ dendritic cells were more densely accumulated in the follicle and the serosal side of Peyer patches, whereas no notable differences in the distribution of CD3e+ T cells and B220+ B cells in the overall regions of Peyer patches were observed (supplemental Figure 9). Thus, there was a defect in the egress of the dendritic cells via the regressed lymphatic vessels of Peyer patches in the LYVE-1-Cre/iDTR mice.
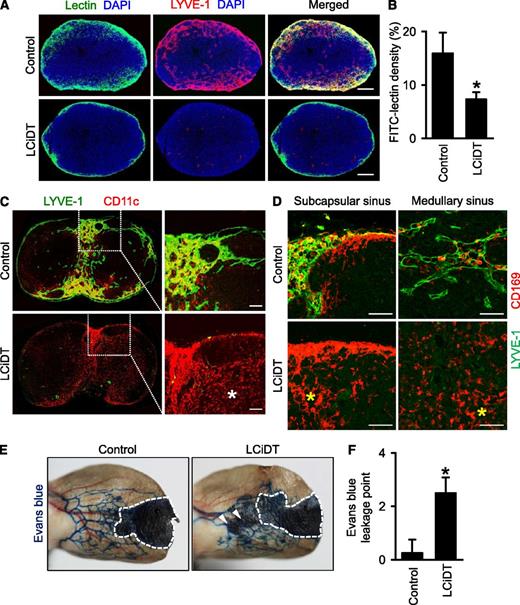
Loss of lymphatic vessels in the lymph nodes of LYVE-1-Cre/iDTR mice causes disrupted trafficking of the dendritic cells and macrophages
The other striking finding in LYVE-1-Cre/iDTR mice was the complete ablation of the lymphatic vessels in the lymph nodes at 24 hours after DT administration (Figure 1C-D; supplemental Figure 1). Time series analyses after DT administration revealed that significant reduction of lymphatic vessels was already detectable at 6 hours and subsequently marked reduction of lymphatic vessels was noted in lymph nodes at 12 hours after DT administration, whereas no overt changes were detected in CD31+ blood vessels at any time point (supplemental Figure 10A-B). In accordance with this finding, functional assays revealed that drainage of lymph flow was limited to the subscapular sinusoidal lymphatic vessels in LYVE-1-Cre/iDTR mice, whereas the flow was well drained into medullary B- and T-cell zones in the control mice (Figure 4A-B). We interpreted the preservation of lymph flow drainage into the lymphatic vessels as follows: Although the LYVE-1+ lymphatic endothelial cells in the sinusoidal lymphatic vessels were completely ablated, the lymphatic sinus consistent with the outer capsule and inner layer could still be patent as a nonspecific conduit for the drainage from the footpad.
Abnormal lymphatic functions of LYVE-1-Cre/iDTR mice after DT administration. Comparisons between control and LCiDT mice for major findings at 24 hours after DT administration. Each group, n = 4–5. *P < .05. (A) Popliteal lymph nodes after injection of fluorescein isothiocyanate–conjugated lectin into the footpad. Scale bars, 200 μm. (B) Quantification of the lectin-drained area density inside the popliteal lymph nodes. (C) Distributions of CD11c+ dendritic cells inside of the inguinal lymph nodes. Indicated areas (square with white dotted line) are magnified in the right panel. The white asterisk indicates abnormally distributed CD11c+ dendritic cells in the B-cell zone. Scale bars, 100 μm. (D) Distributions of CD169+ sinusoidal macrophages in the subscapular and medullary sinuses of the inguinal lymph nodes. The yellow asterisks indicate abnormally distributed CD169+ sinusoidal macrophages. Scale bars, 100 μm. (E) Lymphatic drainage of Evans blue dye in the ear skins. Inside dotted line, Evans blue injection site. The arrowhead indicates leakage areas of skin lymphatic vessels. (F) Quantification of the number of leakage points of Evans blue dye. DAPI, 4,6 diamidino-2-phenylindole.
Abnormal lymphatic functions of LYVE-1-Cre/iDTR mice after DT administration. Comparisons between control and LCiDT mice for major findings at 24 hours after DT administration. Each group, n = 4–5. *P < .05. (A) Popliteal lymph nodes after injection of fluorescein isothiocyanate–conjugated lectin into the footpad. Scale bars, 200 μm. (B) Quantification of the lectin-drained area density inside the popliteal lymph nodes. (C) Distributions of CD11c+ dendritic cells inside of the inguinal lymph nodes. Indicated areas (square with white dotted line) are magnified in the right panel. The white asterisk indicates abnormally distributed CD11c+ dendritic cells in the B-cell zone. Scale bars, 100 μm. (D) Distributions of CD169+ sinusoidal macrophages in the subscapular and medullary sinuses of the inguinal lymph nodes. The yellow asterisks indicate abnormally distributed CD169+ sinusoidal macrophages. Scale bars, 100 μm. (E) Lymphatic drainage of Evans blue dye in the ear skins. Inside dotted line, Evans blue injection site. The arrowhead indicates leakage areas of skin lymphatic vessels. (F) Quantification of the number of leakage points of Evans blue dye. DAPI, 4,6 diamidino-2-phenylindole.
To identify the effect of lymphatic ablation on the immune cell distribution in the lymph nodes, we performed immunostaining for CD11c+ dendritic cells, CD169+ sinusoidal macrophages,26 T cells, and B cells. In the control mice, the dendritic cells and CD169+ sinusoidal macrophages were distributed along the subcapsular and medullary sinusoidal lymphatic vessels in the lymph nodes, but the dendritic cells and macrophages were abnormally and randomly distributed regardless of sinusoidal lymphatic vessels in LYVE-1-Cre/iDTR mice (Figure 4C-D). However, the distributions of T cells and B cells in the lymph nodes of LYVE-1-Cre/iDTR mice were not notably changed at 24 hours after DT administration (supplemental Figure 10C). Thus, loss of lymphatic vessels in LYVE-1-Cre/iDTR mice causes disrupted trafficking of dendritic cells and sinusoidal macrophages. We also identified leakage and misrouting in lymph drainage in the ear skins of LYVE-1-Cre/iDTR mice, although they morphologically appeared normal at 24 hours after DT administration (Figure 4E-F), indicating that functional disturbance takes place in advance of detectable morphologic ablation in the lymphatic vessels.
The major cause of death in LYVE-1-Cre/iDTR mice is not a defect in LYVE-1+ macrophages
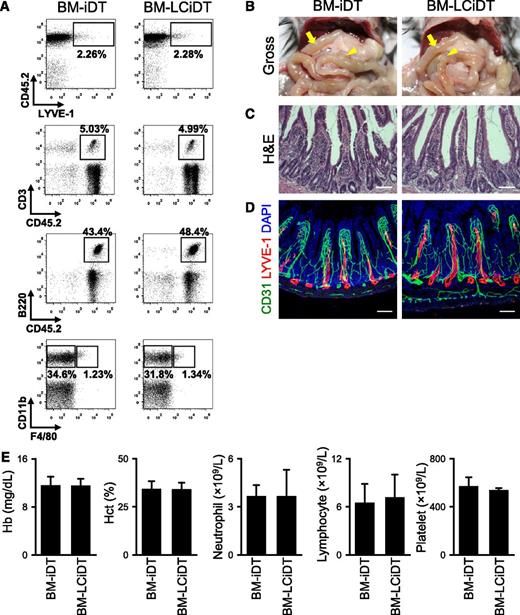
To assess the contribution of LYVE-1+ macrophages27,28 to the cause of death in LYVE-1-Cre/iDTR mice, we tried to selectively ablate LYVE-1+ macrophages using bone marrow transplantation of LYVE-1-Cre/iDTR into the irradiated mice (supplemental Figure 11). The mice receiving bone marrow transplantation from LYVE-1-Cre/iDTR were designated as “BM-LCiDT mice,” and those receiving bone marrow transplantation from iDTR were designated as “BM-iDT mice.” However, the population of LYVE-1+ macrophages in BM-LCiDT mice did not differ from that of BM-iDT mice at 24 hours after DT administration (Figure 5A), which is similar to previous findings.29 In fact, the DT-induced ablation of macrophage-lineage cells required more than 3 days.29 Moreover, no apparent phenotypes related to general appearance, swelling, and hematoma of the small intestine or blood cell counts were detected in BM-LCiDT mice compared with BM-iDT mice (Figure 5B-E). Although we failed to ablate LYVE-1+ macrophages using the BM-LCiDT in 24 hours, we noted that the major cause of death in LYVE-1-Cre/iDTR mice was not related to LYVE-1+ macrophages.
No apparent phenotypes are detected in BM-LCiDT mice at 24 hours after DT administration. (A) Comparisons of flow cytometric profiles of LYVE-1+/CD45+ macrophages, CD3+/CD45+ T lymphocytes, B220+/CD45+ B lymphocytes, CD11b+/F4/80+ macrophages, and CD11b+/F4/80− monocytes in the peripheral bloods. Note that there are no differences in the population of each immune cell between BM-iDT and BM-LCiDT. (B) Gross images of the abdominal contents. The arrow indicates the proximal portion of the small intestine, and the arrowhead indicates the Peyer patch. (C) Intestinal villi in the duodenum stained with hematoxylin and eosin. Scale bars, 100 μm. (D) LYVE-1+ lymphatic vessels and CD31+ blood vessels in jejunums. Scale bars, 100 μm. Similar findings were observed in 3 to 4 different mice. (E) Blood profiles of hemoglobin, hematocrit, neutrophils, lymphocytes, and platelets. Each group, n = 4 to 5. DAPI, 4,6 diamidino-2-phenylindole; H&E, hematoxylin and eosin.
No apparent phenotypes are detected in BM-LCiDT mice at 24 hours after DT administration. (A) Comparisons of flow cytometric profiles of LYVE-1+/CD45+ macrophages, CD3+/CD45+ T lymphocytes, B220+/CD45+ B lymphocytes, CD11b+/F4/80+ macrophages, and CD11b+/F4/80− monocytes in the peripheral bloods. Note that there are no differences in the population of each immune cell between BM-iDT and BM-LCiDT. (B) Gross images of the abdominal contents. The arrow indicates the proximal portion of the small intestine, and the arrowhead indicates the Peyer patch. (C) Intestinal villi in the duodenum stained with hematoxylin and eosin. Scale bars, 100 μm. (D) LYVE-1+ lymphatic vessels and CD31+ blood vessels in jejunums. Scale bars, 100 μm. Similar findings were observed in 3 to 4 different mice. (E) Blood profiles of hemoglobin, hematocrit, neutrophils, lymphocytes, and platelets. Each group, n = 4 to 5. DAPI, 4,6 diamidino-2-phenylindole; H&E, hematoxylin and eosin.
Ablation of the dermal lymphatic vessels is feasible by intradermal administration of DT in LYVE-1-Cre/iDTR mice
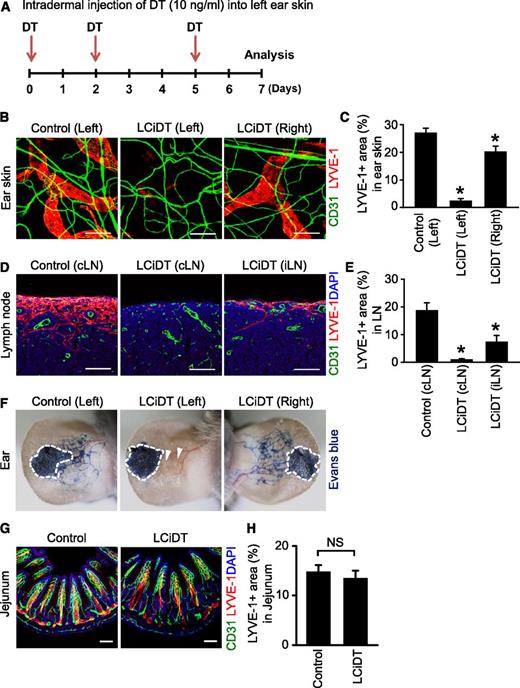
Because such an early death by sepsis limited a detailed investigation on the roles of lymphatic vessels in an organotypic manner, we tried to ablate the skin dermal lymphatic vessels by intradermal administration of DT (10 ng) into the left ear skin 3 times at 2- to 3-day intervals in LYVE-1-Cre/iDTR mice (Figure 6A). The LYVE-1-Cre/iDTR mice survived for 7 days without visible phenotypes under this local ablative approach. Intriguingly, the lymphatic vessels of the left ear skin and draining cervical lymph node in LYVE-1-Cre/iDTR mice were almost completely ablated and their lymph drainage completely disappeared, whereas the lymphatic vessels of the contralateral right ear skin and distant inguinal lymph node in LYVE-1-Cre/iDTR mice were slightly and moderately reduced and their lymph drainage was relatively intact (Figure 6B-F). Nevertheless, the intestinal lacteals in LYVE-1-Cre/iDTR were not altered (Figure 6G-H). Thus, local ablation of the lymphatic vessels could be feasible to uncover roles of the lymphatic vessels using this mouse model system.
Local ablation of lymphatic vessels after intradermal injection of DT into the ear skin. (A) Diagram of experimental scheme for local lymphatic ablation by intradermal injection of DT (10 ng/mL) into the left ear skin of LCiDT mice. Samples were harvested at 7 days after triple injections of DT. (B) Images showing LYVE-1+ lymphatic vessels and CD31+ blood vessels in the left and right ear skins of LCiDT and control mice. Scale bars, 100 μm. (C) Quantifications for LYVE-1+ area in the ear skins. Each group, n = 4. *P < .05 vs control. (D) Images showing LYVE-1+ lymphatic vessels in draining cervical lymph node (cLN) and distal inguinal lymph node (iLN) of LCiDT and control mice. Scale bars, 100 μm. (E) Quantifications for LYVE-1+ area in the LNs. Each group, n = 4. *P < .05 vs cLN of control. (F) Lymphatic drainage of Evans blue dye in the ear skins. The dotted line shows the Evans blue injection site. The arrowhead indicates no lymph drainage. Similar findings were observed in 3 to 4 different mice. (G) Images showing LYVE-1+ lymphatic vessels in jejunums. Scale bars, 100 μm. (H) Quantifications for LYVE-1+ area in the jejunums. Each group, n = 4. NS, not statistically significant. DAPI, 4,6 diamidino-2-phenylindole.
Local ablation of lymphatic vessels after intradermal injection of DT into the ear skin. (A) Diagram of experimental scheme for local lymphatic ablation by intradermal injection of DT (10 ng/mL) into the left ear skin of LCiDT mice. Samples were harvested at 7 days after triple injections of DT. (B) Images showing LYVE-1+ lymphatic vessels and CD31+ blood vessels in the left and right ear skins of LCiDT and control mice. Scale bars, 100 μm. (C) Quantifications for LYVE-1+ area in the ear skins. Each group, n = 4. *P < .05 vs control. (D) Images showing LYVE-1+ lymphatic vessels in draining cervical lymph node (cLN) and distal inguinal lymph node (iLN) of LCiDT and control mice. Scale bars, 100 μm. (E) Quantifications for LYVE-1+ area in the LNs. Each group, n = 4. *P < .05 vs cLN of control. (F) Lymphatic drainage of Evans blue dye in the ear skins. The dotted line shows the Evans blue injection site. The arrowhead indicates no lymph drainage. Similar findings were observed in 3 to 4 different mice. (G) Images showing LYVE-1+ lymphatic vessels in jejunums. Scale bars, 100 μm. (H) Quantifications for LYVE-1+ area in the jejunums. Each group, n = 4. NS, not statistically significant. DAPI, 4,6 diamidino-2-phenylindole.
Discussion
In our study, we generated the LYVE-1-Cre/iDTR mouse, which enabled ablation of LYVE-1+ lymphatic vessels in a DT-dependent manner. Using this model, we intended to clarify overall lymphatic functions as well as determine the regenerative ability of the lymphatic vessels. However, unexpectedly, all of the LYVE-1-Cre/iDTR mice died within 60 hours after DT administration. To determine the cause of lethality in the LYVE-1-Cre/iDTR mice, we carefully analyzed the changes in lymphatic vessels in most of the organs at 24 hours after DT administration. Strikingly, LYVE-1+ lacteals in the small intestine and LYVE-1+ lymphatic vessels in all lymph nodes had mostly disappeared, whereas LYVE-1+ lymphatic vessels in the other organs were morphologically normal. We attribute these changes to a cause of death of the LYVE-1-Cre/iDTR mice on DT administration, and these findings uncover defensive roles of lymphatic vessels in intestinal villi and lymph nodes.
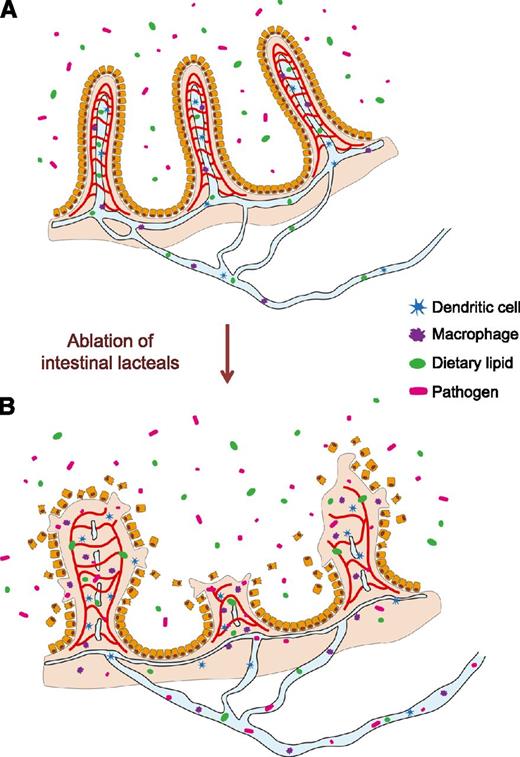
Intestinal villi are exposed to digested and undigested foods, pathogens, and residential microorganisms including E coli. Structural integrity and local immune surveillance of intestinal villi are essential to protect the body from pathogen invasion through the intestine.9,30,31 In our findings, the disappearance of lacteals appears to trigger distortion of blood capillaries and the whole architecture of the villi, which allows invasion of intestinal pathogens including E coli, without barrier and immune surveillance, and subsequently causes severe inflammation in the intestine (Figure 7). Considering that the common features of septic shock were detected in the LYVE-1-Cre/iDTR mice within 24 hours after DT administration, we assume that the pathogen invasion could occur through either damaged intestinal lymphatic vessels before their complete loss or damaged intestinal blood vessels just after DT administration. This cascade of pathologic changes consequently could lead to a catastrophic death by systemic infection and septic shock via random invasion of intestinal pathogens into the systemic circulation.31 Lymphatic vessels in the intestinal villi are actively involved in transport of absorbed dietary lipids as a form of colloidal lipoproteins and certain highly lipophilic compounds (eg, lipid-soluble vitamins and drugs) from absorptive epithelial cells to the systemic circulation via mesenteric collecting lymphatic vessels.7 Therefore, the possible malnutrition caused by acute suppression of absorption and draining for the digested and processed foods in the intestinal villi7,32 could exacerbate the survival duration of LYVE-1-Cre/iDTR mice as time progresses. In fact, such septic features and consequences in the LYVE-1-Cre/iDTR mice are very similar to those in the high-grade sepsis mouse model generated by extensive cecal ligation and puncture.33 Thus, LYVE-1-Cre/iDTR mice can be used as an alternative severe sepsis model.
Schematics depicting defensive roles of lymphatic vessels in the small intestine. In the control mice (A), the intestinal lymphatic vessels play critical roles not only in draining absorbed lipids but also in the maintenance of villi structure and function. In the LYVE-1-Cre/iDTR mice (B), ablation of intestinal lacteals causes disruptions of blood vessels and villi architecture, which leads to invasion of intestinal pathogens into the circulatory system via the blood vessels.
Schematics depicting defensive roles of lymphatic vessels in the small intestine. In the control mice (A), the intestinal lymphatic vessels play critical roles not only in draining absorbed lipids but also in the maintenance of villi structure and function. In the LYVE-1-Cre/iDTR mice (B), ablation of intestinal lacteals causes disruptions of blood vessels and villi architecture, which leads to invasion of intestinal pathogens into the circulatory system via the blood vessels.
Lymph nodes contain a network of lymphatic vessels that are conduits for passages of pathogens, immune cells, metastatic tumor cells, and lymph fluid. The microstructure of lymphatic vessels in lymph nodes consists of sinusoidal extensions that begin at the peripheral afferent lymphatic vessels, are further distributed just below the capsule and surrounding B-cell follicles, and stretch toward the medullary region including the T-cell follicular zone of lymph nodes.34,35 Lymphatic vessels in lymph nodes play a key role in the routing and trafficking of immune cells including pathogen-carrying CD11c+ dendritic cells and CD169+ sinusoidal macrophages for adaptive immunity.4,5,12,36 Our study shows that the complete ablation of lymphatic vessels in the lymph nodes hampered the drainage of lymph flow limited to only the subscapular lymphatic vessels and caused abnormal distribution of the dendritic cells and macrophages along the subcapsular and medullary sinusoidal lymphatic vessels in the lymph nodes. In addition, ablation of lymphatic vessels in the Peyer patches also impaired dendritic cell egress to the connecting mesenteric lymph nodes. Thus, losses of lymphatic vessels in the lymph nodes and Peyer patches of LYVE-1-Cre/iDTR mice cause disrupted trafficking and egress of dendritic cells and macrophages, leading to subsequent loss of clearing function for draining pathogens from peripheral tissues. In particular, loss of lymphatic vessels in the mesenteric lymph nodes could allow the pathogens drained from the damaged intestinal lumen to transfer directly to the systemic circulation without proper filtering and clearing in the LYVE-1-Cre/iDTR mice, which could also contribute to septic shock and lethality.
Furthermore, we found that functional disturbance occurs in advance of detectable morphologic ablation in lymphatic vessels through the functional assay in the ear skin. These functional disturbances also contribute to accelerated death from defective pathogen transport from the periphery to the lymph nodes and impairment of pathogen clearance in the lymph nodes, leading to improper adaptive immunity.4-6,37
Although our approach to systemic ablation of the lymphatic vessels unveiled the defensive roles of lymphatic vessels, the early death by sepsis limited a detailed investigation on the roles of lymphatic vessels in an organotypic manner. To overcome this issue, we performed local intradermal administration of DT into the LYVE-1-Cre/iDTR mice and achieved selective ablation of lymphatic vessels in the DT-injected region of the skin and its draining lymph nodes. By using this approach, we observed that local ablation of lymphatic vessels could be achievable to unveil roles of the lymphatic vessels in an organotypic manner as a future study. One of the intriguing findings of our study is the differential sensitivity among organs in the DT-induced ablation of LYVE-1+ lymphatic vessels. Considering that there were no differences in the degrees of LYVE-1 expression and turnover rates of lymphatic endothelial cells among the organs we analyzed, we believe that the heterogeneity of intrinsic properties in lymphatic endothelial cells among organs could to be a possible factor for the differential sensitivity, which is gradually being elucidated.38,39
In summary, we identified additional and novel functions of lymphatic vessels in the small intestine and lymph nodes using a LYVE-1-Cre/iDTR mouse model. Intestinal lymphatic vessels, including lacteals, play a critical role in the maintenance of the structure and function of villi. Ablation of intestinal lacteals causes disruptions of blood vessels and villi architecture, which leads to invasion of intestinal pathogens into the circulatory system and subsequent septic shock and death. Moreover, intact lymphatic vessels in lymph nodes are critical for distribution and trafficking of dendritic cells and macrophages for regulation of proper adaptive immunity.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Su-Jin Seo for technical assistance.
This work was supported by grants from the National Research Foundation (R2009-0079390, GYK) and a grant from the World Class University (R31-2009-000-10071-0) funded by the Ministry of Education, Science and Technology, Korea.
Authorship
Contribution: J.Y.J., Y.J.K., S.-H.L., J.L., and K.H.K. designed and performed the experiments, analyzed the data, generated the figures, and wrote the manuscript; and D.K., G.Y.K., and O.J.Y. designed, organized, and supervised the study, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gou Young Koh, Graduate School of Biomedical Science and Engineering, Korea Advanced Institute of Science and Technology, Daejeon, 305-701, Republic of Korea; e-mail: gykoh@kaist.ac.kr; and Ook Joon Yoo, Graduate School of Biomedical Science and Engineering, Korea Advanced Institute of Science and Technology, Daejeon, 305-701, Republic of Korea; e-mail: ojyoo@kaist.ac.kr.
References
Author notes
J.Y.J. and Y.J.K. contributed equally to this study.