In this issue of Blood, Nogai et al have identified the atypical nuclear factor–κB (NF-κB) modulator IκBζ as a key factor that drives oncogenic NF-κB activity in an activated B-cell subtype of diffuse large B-cell lymphoma (ABC DLBCL).1
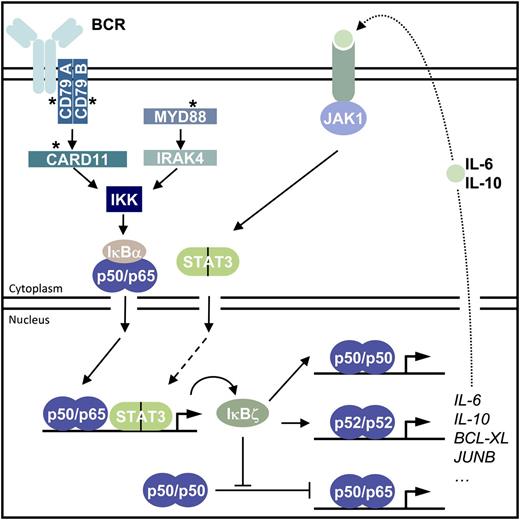
Scheme depicting the regulation and biological effects of IκBζ in ABC DLBCL. Somatic oncogenic mutations (asterisks) in the BCR signaling pathway (CD79A, CD79B, and CARD11) or in MYD88 lead to constitutive canonical IKK/NF-κB activation and promote IκBζ expression in ABC DLBCL cells. IκBζ contributes to expression of the NF-κB gene signature that includes survival factors (eg, BCL-XL) as well as the cytokines IL-6 and IL-10. JAK1-STAT3 is activated by autocrine action of IL-6 and IL-10. As STAT3 is able to induce IκBζ expression, the data indicate the potential existence of an autoregulatory feed-forward loop to enhance IκBζ expression and survival function. Mechanistically, IκBζ bind to p50 and p52 and can directly confer transactivation potential to these transcriptionally inactive homodimers. Alternatively, IκBζ may displace p50 (or also p52) homodimers to allow promoter occupancy of active NF-κB p50/p65 heterodimers. BCR, B-cell receptor.
Scheme depicting the regulation and biological effects of IκBζ in ABC DLBCL. Somatic oncogenic mutations (asterisks) in the BCR signaling pathway (CD79A, CD79B, and CARD11) or in MYD88 lead to constitutive canonical IKK/NF-κB activation and promote IκBζ expression in ABC DLBCL cells. IκBζ contributes to expression of the NF-κB gene signature that includes survival factors (eg, BCL-XL) as well as the cytokines IL-6 and IL-10. JAK1-STAT3 is activated by autocrine action of IL-6 and IL-10. As STAT3 is able to induce IκBζ expression, the data indicate the potential existence of an autoregulatory feed-forward loop to enhance IκBζ expression and survival function. Mechanistically, IκBζ bind to p50 and p52 and can directly confer transactivation potential to these transcriptionally inactive homodimers. Alternatively, IκBζ may displace p50 (or also p52) homodimers to allow promoter occupancy of active NF-κB p50/p65 heterodimers. BCR, B-cell receptor.
Sustained activation of NF-κB survival signaling is a hallmark of ABC DLBCL. Previous work has largely focused on the identification of oncogenic mutations and molecular mechanisms in the cytoplasm that contribute to deregulated activation of the NF-κB signaling pathway.2 By identifying nuclear IκBζ (also termed MAIL) as an essential survival factor in the majority of ABC DLBCL cells, the current study of Nogai, Lenz, and colleagues highlights the importance of also shaping an oncogenic transcriptional NF-κB response inside of the nucleus.1
The NF-κB family comprises the subunits NF-κB1/p50, NF-κB2/p52, p65/RelA, c-Rel, and RelB that can form various homodimers and heterodimers with distinct biological properties.3 In contrast to the cytosolic NF-κB inhibitors IκBα, IκBβ, or IκBε, the atypical IκB protein IκBζ is regulating NF-κB exclusively in the nucleus. After stimulation of innate immune or cytokine receptors in macrophages, IκBζ expression is induced and it activates a subset of NF-κB target genes including IL-6 by selectively enhancing transcriptional activity of p50 homodimers.4,5 In this issue, the authors demonstrate that IκBζ is highly expressed in the majority of ABC DLBCL cell lines and patient samples and contributes to the induction of the NF-κB target gene signature (see figure).1 IκBζ depletion induces toxicity in ABC DLBCL cells, but not in other NF-κB–dependent or –independent lymphomas. Mechanistically, IκBζ promotes transcriptional activity of p50 and p52 homodimers; in ABC DLBCL cells, IκBζ binds exclusively to the p50 and p52 subunits of NF-κB. Because p50 or p52 do not contain transcriptional activation domains, the data suggest that IκBζ confers transactivating potential to allow induction of a large number of NF-κB target genes in ABC DLBCL cells (see figure). Also, it is conceivable that IκBζ may facilitate recruitment of transcriptionally active NF-κB heterodimers by displacing inactive p50 and p52 homodimers. In any case, the strong effects on ABC DLBCL viability after knockdown underscore that IκBζ is a key driver of pathological NF-κB transcription inside of the nucleus.
As IκBζ does not confer catalytic activity, it may not be a direct target for pharmacologic inhibition. Nevertheless, the data suggest that strategies to reduce IκBζ protein levels could be beneficial for ABC DLBCL therapy. Emphasizing this notion, the study reveals interesting insights into the regulation of IκBζ expression in the tumor cells (see figure). Previous data demonstrated that IκBζ is not present in resting cells, but expression is highly induced upon stimulation of Toll-like receptors or interleukin 1 (IL-1) receptor by a myeloid differentiation protein 88 (MYD88)– and IL-1 receptor-associated kinase 4 (IRAK4)–dependent pathway.5,6 Congruent with a critical function of canonical NF-κB signaling, oncogenic MYD88 or caspase recruitment domain-containing protein 11 (CARD11) variants trigger IκBζ expression in ABC DLBCL.1 However, constitutive NF-κB activation may not be sufficient for full IκBζ induction because IκBζ expression is low in Hodgkin lymphoma or multiple myeloma, despite the fact that survival of both lymphomas relies on NF-κB. Interestingly, signal transducer and activator of transcription 3 (STAT3) activation can also induce the expression of IκBζ.7,8 In ABC DLBCL, STAT3 is phosphorylated and activated by an autocrine loop that involves the secretion of the cytokines IL-6 and IL-10 (see figure).2 Importantly, expression of both cytokines is under control of IκBζ and, consequently, IκBζ knockdown severely diminishes STAT3 phosphorylation.1 Even though it remains to be shown whether STAT3 also directly influences IκBζ in ABC DLBCL, the data imply that IκBζ, IL-6/IL-10, and STAT3 could constitute a vicious autoregulatory feed-forward cycle that contributes to the maintenance of deregulated oncogenic STAT3 and NF-κB activity in the nucleus of ABC DLBCL. Of note, the ABC DLBCL tumors that have highest STAT3 activity also display higher expression of the NF-κB gene signature, and nuclear IκBζ could provide a missing link for this phenomenon.9 Furthermore, Janus kinase (JAK) inhibitors acting upstream of STAT3 synergize with IκB kinase (IKK) inhibitors in killing ABC DLBCL cells. It will interesting to see if JAK inhibition directly impacts IκBζ expression.
Taken together, IκBζ is a key regulator of the NF-κB prosurvival network in ABC DLBCL. The ABC DLBCL subgroup comprises ∼40% of all DLBCL, and with a 3-year progression-free survival of <50%, it remains one of the clinically most relevant lymphoma entities. Future studies will need to elucidate whether high IκBζ protein levels could potentially serve as a diagnostic or prognostic marker for DLBCL and determine how pharmacologic strategies that reduce IκBζ expression may be used for therapeutic intervention.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal